Materials Science: Unit I: Crystallography
Polymorphism
Definition, Solved Example Problems | Crystallography
Many substances exist in more than one stable crystalline form.
POLYMORPHISM Many substances exist in more than one stable crystalline form. The various forms have the same composition but the crystal structures are different. The changes in crystal structure is caused due to either a change in temperature or pressure or both. The ability of a material to exist in two or more crystal structure is called polymorphism. The polymorphism is observed in pure elements and chemical compounds both in organic and inorganic compounds. If the structural change is reversible, then the polymorphic change is known as allotropy. This means that during this process, only physical properties change with no change in the chemical properties. Cobalt at ordinary temperature has HCP structure but while it is heated above 477°C, it changes to FCC structure. A good example of polymorphism and allotropy is iron (Fe). This can be understood from the following reaction When the iron is heated to 912°C, the structure changes to FCC and when it is cooled it returns back to BCC structure. Similarly when iron at iron at 912°C is heated to to beyond 1400°C, it converts to BCC structure and returns to FCC structure on cooling. A similar reaction occurs in diamond and graphite. It is well known that carbon exists in either diamond or graphite form. Diamond is very hard, transparent and an electrical insulator while graphite is a good conductor and it is a lubricant. Note: These changes in their properties are due to their differences in their structure and bonding Almost all the properties change, when a metal or alloy changes from one allotrope to another. Some of the properties which changes are: (a) Specific volume (b) Packing density (c) Electricity conductivity (d) Thermal conductivity (e) Mechanical properties (f) Magnetic properties (g) Chemical properties (h) Physical propertiesDefinition
Example - 1
Example - 2
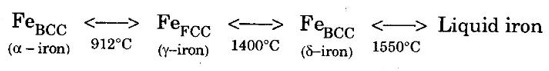
Example - 3

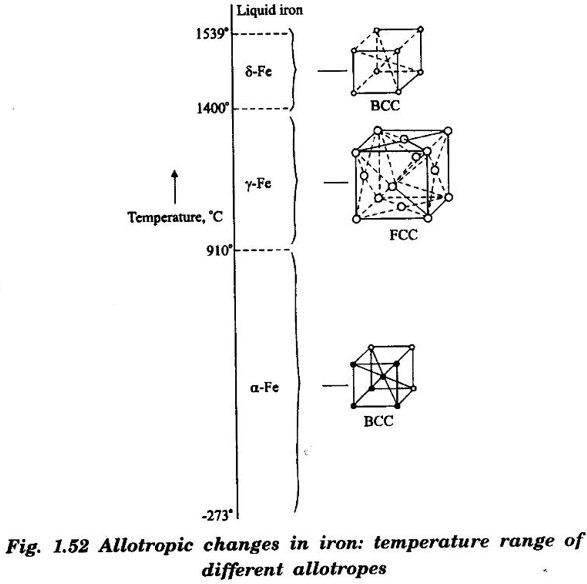
Materials Science: Unit I: Crystallography : Tag: : Definition, Solved Example Problems | Crystallography - Polymorphism
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
