Materials Science: Unit I: Crystallography
Phase changes or Transformations
Crystallography
Phase transformation is an important process in the production and treatment of metallic compounds and alloys.
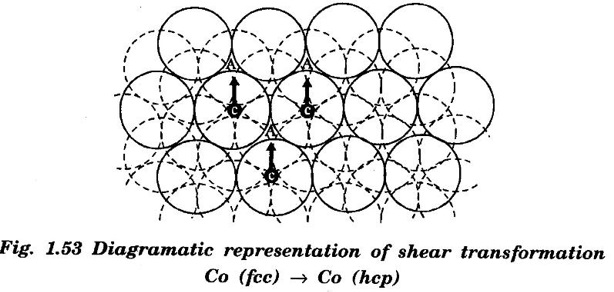
PHASE CHANGES OR TRANSFORMATIONS Phase transformation is an important process in the production and treatment of metallic compounds and alloys. It involves nucleation and growth. Phase transformation occurs due to change of pressure, temperature or composition of the alloy. The rate of phase transformation influences the microstructure of the new phase formed. An alloy in a phase can spontaneously transform to β phase only if Gα > Gβ (Gα, Gβ - Gibbs, free energy is for α, β phases respectively. These processes can take place through distinctly different types of phase transformations. The properties of the final phase depend on thermal and mechanical treatments. Major types of phase transformations are the following: In this type of phase transformation, a single component material crystallizes from liquid state with no change in composition e.g. crystallization of copper from melt. The chemical formula of the material remains unchanged under such transformations. Congruent transformation can be two types: • Reconstructive transformation, • Displacive transformation. In reconstructive phase transformation, the atoms diffuse across the α - β interface to transform α phase into β phase. The phase change occurs as a gradual process in which the atomic coordination is altered. The atoms of α-phase rearrange to form only β-phase but also intermediate phases β', β" etc. There other subsequent reconstructive transformations through which β', β" phases are converted to more stable β phase. This type of transformation requires high activation energy since there would be a significant rearrangement of bonds. Examples of reconstructive transformation are polymorphic transformation from α -Ti (hcp) to β - Ti (bcc), cristobolite to tridymite etc. A displacive transformation arises from the cooperative movements of a large number of neighbouring atoms. The example of such transformation is This type of transformation occurs very rapidly since no bonds are broken during the process. During the shear transformation, there is shear like cooperative displacement of one layer of atoms with respect to the next layer. These layers are close-packed planes. The transformation of Co (fcc) to Co (hcp) is an example of this type of transformation. The atoms of (111) planes of fcc Co move through a fraction of interatomic distance to give hcp stacking during this transformation, fig. 1.53. Consequently, ABCABC... stacking of fcc changes to ABAB... stacking of hcp. The atoms of C-layer in fcc stacking move together such that these atoms are arranged on A-valleys i.e. they form A-layer. The A-layer atoms are displaced in a cooperative manner such that they form B-layer and B-layer atoms form A-layer etc. The solidification of metals and alloys is an important industrial process. The most metals are melted and then casted into a semifinished or finished shape. The solidification of a metal or alloy takes place in following two steps: 1. The formation of stable nuclei in the melt (nucleation). 2. The growth of nuclei into crystals and the formation of a grain structure.Different types of phase transformations
(i) Congruent transformation
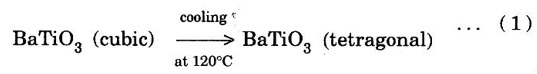
(ii) Shear transformation

Solidification of metals and alloys
Materials Science: Unit I: Crystallography : Tag: : Crystallography - Phase changes or Transformations
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
