Materials Science: Unit I: Crystallography
Homogeneous or Self-Nucleation
Crystallography
The formation of nuclei in a melt without the aid of impurity foreign particles is called homogeneous or self-nucleation.
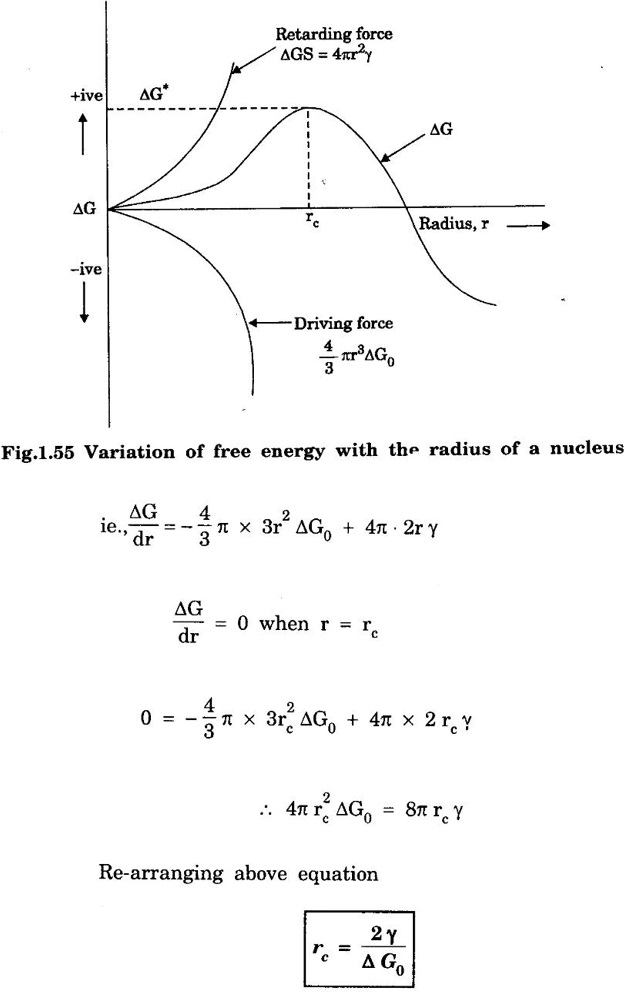
HOMOGENEOUS OR SELF-NUCLEATION The formation of nuclei in a melt without the aid of impurity foreign particles is called homogeneous or self-nucleation. This nucleation occurs in perfectly homogeneous materials such as pure molten metals. The liquid (molten) metal provides atoms to the formation of nuclei. The molten metal is cooled below its freezing temperation during the nucleation. During the nucleation, the molten metal must be cooled below its freezing temperature. The atoms in a molten liquid bond together to form a small crystal called 'embryo'. The embryo grows into a nucleus with the addition of atoms. In homogeneous nucleation the stability of a nucleus is controlled by two factors (i) Free energy change during the liquid solid transition. (ii) Surface energy the nucleus thus formed. As a result, the total free energy change associated with the embryo formation is given by where, ΔG - free energy change between liquid and solid per unit volume r - radius of an embryo (nucleus) considered to be spherical γ - specific surface energy Below the melting temperature Ts, Δ G0 is negative and hence the first term on the right of equation (1) is negative. Since γ is always positive, the second terms representing the surface energy between the embryo and the matrix is positive. The term 4/3 π r3 Δ G0 is the driving force for the creation of solid -liquid interface. This term decreases (Δ G0 is negative) as r2 increases. However, the surface energy which is the retarding force increases as r2 increases. Fig.1.55 shows a plot of free energy Δ G versus the nucleus radius r. The maximum Δ G equal to Δ G for r = rc. where rc is the critical radius of the nucleus. At the critical radius, the volume free energy decrease, just equals the surface free energy of the nucleus. when r > rc the free energy of the system decreases and initiating the formation of crystal. It is noted that for a nucleus of a size less than the critical value rc the free energy of the system increases. This is due to the increment of free energy by the formation of a new interface exceeds reduction in free energy by the formation of a nucleus. Therefore, a nucleus of a size less than rc cannot grow and soon dissolved in the liquid metal. If a nucleus appears with a size exceeding rc, it will be stable. It will be capable of growth because the free energy of the system reduces as the size of the nucleus increases.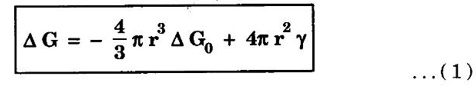
Materials Science: Unit I: Crystallography : Tag: : Crystallography - Homogeneous or Self-Nucleation
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
