Materials Science: Unit I: Crystallography
Heterogeneous Nucleation
Crystallography
Most frequently, the source about which nuclei are formed on solid particles are always present in the melt.
HETEROGENEOUS NUCLEATION Most frequently, the source about which nuclei are formed on solid particles are always present in the melt. If the particles of the impurity have a crystal structure closely resembling that of the solidifying metal, then they play the part of ready centers of crystallization. Such surfaces or impurities are "wet" by both liquid and solid, lowering the net energy associated with the formation of nucleus. Fig. 1.56 illustrates mechanism of heterogeneous nucleation. The heterogeneous nucleus is considered as a spherical cap on a solid, flat substrate. The volume of the cap depends on the contact angle 0 at the nucleus - liquid - substrate junction. If the contact angle is less than 180° a particular surfaced will serve as nucleation catalyst for that system. The change in free energy associated with the formation of the embryo is given by, where, V - volume of the spherical cap (nucleus) ACL - the interfacial surface area between liquid and the cap r - the radius of the cap (nucleus) γCL, γSC and γSL are interfacial surface tension between cap (nucleus) and liquid, cap and substrate and substrate liquid respectively. In homogeneous nucleation, the critical size of the nucleus given by In heterogeneous nucleation, the value of γCL, the interfacial surface tension surface energy is much smaller. Therefore, the critical size of the nucleus is much smaller. As a result, the number of atoms that must be crystallized before the critical radius size (rc) is reached is much less for heterogeneous nucleation than for the homogeneous one. Hence smaller amount of undercooling is required.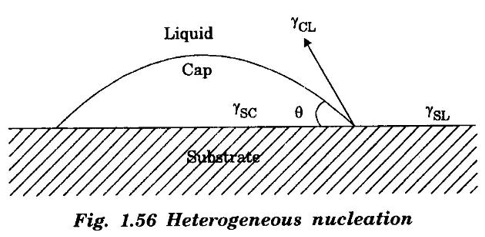
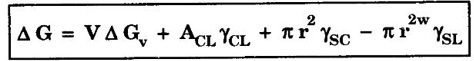
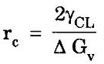 and it is independent of contact angle.
and it is independent of contact angle.
Materials Science: Unit I: Crystallography : Tag: : Crystallography - Heterogeneous Nucleation
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
