Materials Science: Unit I: Crystallography
Crystal Structures
The alkali metals Li, Na, K, etc., have bcc structures, some transition elements and rare earths have fcc structures, elements of second group have hcp structures.
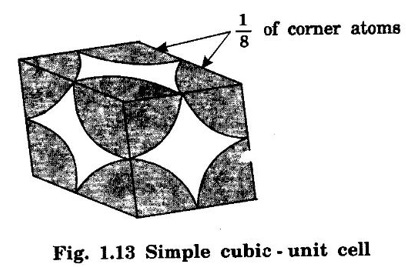
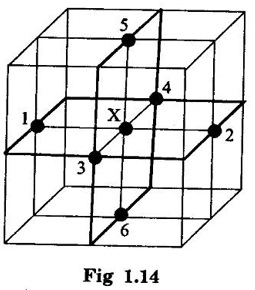
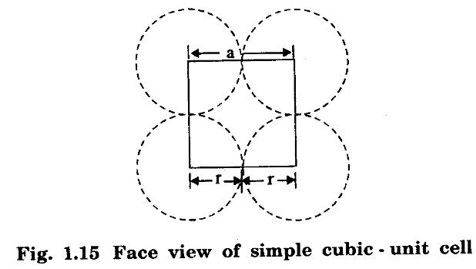
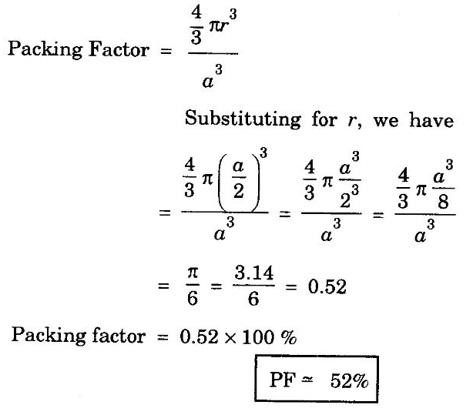
CRYSTAL STRUCTURES COORDINATION NUMBER AND PACKING FACTOR FOR CRYSTAL STRUCTURES SC, BCC, FCC and HCP It is noted that a large number of metallic crystal structures have hcp, fcc and bec structures. Simple Cubic (SC) structure is very rare in metals, The alkali metals Li, Na, K, etc., have bcc structures, some transition elements and rare earths have fcc structures, elements of second group have hcp structures. The simple cubic structure is the simplest and easiest crystal structure. In this structure, there is one atom at each of the '8' corners of unit cell. These atoms touch each other along cube edges. (fig 1.12). The unit cell of a simple cubic structure is shown in fig 1.12. The representative arrangement is drawn in fig. 1.12 (a). The actual way of arrangement is shown in Fig. 1.12 (b). There are 8 atoms, one atom at each corner of unit cell. Each corner atom is shared by 8 adjascent unit cells. (Fig. 1.13) ⸫ Share of each unit cell = 1/8 of corner atoms = 1/8 × 8 = 1 Total number of atoms in one unit cell = 1 atom Simple cubic unit cell has 8 corner atoms. Let us consider one of the corner atoms (say X). It is shared by 8 adjacent unit cells as shown in fig 1.14. There are 4 nearest neighbouring atoms to this particular atom X' which are shown by 1, 2, 3, and 4 in a plane (horizontal plane). Further, there are 2 more nearest atoms, one directly above (atom 5) and the other one directly below (atom 6) the atom X. Thus, there are only six (4+2) nearest neighbours for the atom X. Hence, coordination number for simple cubic is 6. Note: Similarly if we consider any corner atom, the total number of nearest neighbours i.e., the co-ordination number is the same. Consider a face of unit cell of a simple cubic structure (fig 1.15). The atoms touch each other along the edges of the cube. It is clear that the distance between the centres of two nearest atoms is equal to the cube edge a. If 'a' is the side of the unit cell and r its radius, then, from fig. 1.15, 2r = a Number of atoms per unit cell = 1 Volume of the atoms in the unit cell, v = 1 × 4/3 πr3 Atomic radius r = a/2 Total volume of the unit cell, V = a3 We know that packing factor = υ/V Substituting for v and V, we have Thus, 52% of the volume of unit cell is occupied by the atoms and the remaining 48% volume is vacant. Example: Only one element polonium (Po) at certain temperature range exhibits this crystal structure.Simple Cubic (SC) Structure
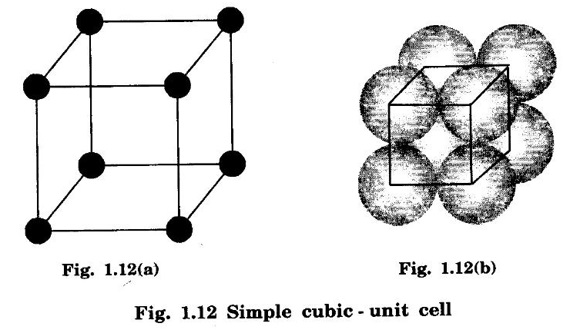
1. Number of atoms per unit cell
2. Coordination number
3. Atomic radius
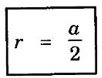
4. Packing factor
Materials Science: Unit I: Crystallography : Tag: : - Crystal Structures
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
