Materials Science: Unit I: Crystallography
Anna University Solved Problems [lattice constant]
Crystallography | Materials Science
Anna University Solved Problems with solution: Crystallography - Materials Science
ANNA UNIVERSITY SOLVED PROBLEMS Problem 1.3 Calculate the lattice constant of Fe. Given: density of iron 7860 kg/m3, atomic weight 55.85 and Avagadro's number 6.023 × 1026 atoms/mol Given data Density of iron ρ = 7860 kg m-3 Atomic weight M = 55.85 Avagadro's number N = 6.023 × 1026 mol-1 Number of atoms per unit cell (BCC) = 2 Solution We know that Substituting the given values, we have Taking cube root on both sides, we have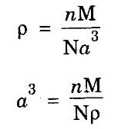
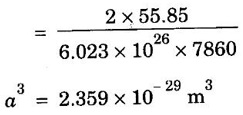
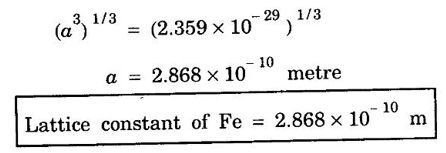
Materials Science: Unit I: Crystallography : Tag: : Crystallography | Materials Science - Anna University Solved Problems [lattice constant]
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
