Materials Science: Unit I: Crystallography
Additional 2 Marks Question & Answers
Crystallography | Materials Science
Additional 2 Marks Question & Answers : Crystallography - Materials Science
ADDITIONAL PART - A Q & A 1. What are crystalline materials? Give example. Crystalline materials are materials in which the atoms are arranged in an orderly fashion throughout in a three dimensional space. Example: Copper, silver, etc., 2. What is an amorphous solid? Give example. It is a type of solid in which the atoms are not arranged in an orderly fashion. (randomly). 3. What is a crystal? A crystal is a three dimensional solid composed of a periodic and regular arrangement of atoms. 4. What are lattice points? The points in the space to represent position of atom or group of atoms of the crystal are called lattice points. 5. What is basis? The crystal structure is formed by associating with every lattice point a unit assembly of atoms or molecules (ie, one or more atoms or molecules). This unit assembly is called the basis or pattern. 6. What are the differences between crystalline and non-crystalline material. 7. What are the lattice parameters of an unit cell? The intercepts on the axes a, b and c and interfacial angles α, β and γ are called lattice parameters of an unit cell. 8. Define inter-atomic distance and interplanar distance. Interatomic distance: It is the distance between the centres of any two nearest atoms. Inter-planer distance: It is the perpendicular distance between any two parallel planes. 9. What is the relation between lattice constant 'a' and density 'ρ' of the crystal? where, n - Number of atoms in unit cell M - atomic weight N - Avagadro's number = 6.023 × 1026 mol-1 10. What is meant by loosely packed crystal structure? Give an example of this type of material. The loosely packed crystal structure has the packing factor less than 0.74. That is, in which more vacant site is available. Simple cubic polonium and body centred cubic sodium are examples for loosely packed crystal structures. 11. What is meant by closely packed structure? Give one example for this. Closely packed structure has the highest packing factor of 0.74. Here the atoms are closely packed leaving a small space as vacant site in the crystal. Face centred cubic copper and hexagonal close packed magnesium are examples to this closely packed structure. 12. What is crystal defect? The deviation from the regularity of arrangement of atoms is called crystal imperfection or crystal defect. 13. What various types of defects. (i) Point defects (zero dimensional) (a) Impurity defect (i) Substitutional impurity defect (ii) Interstitial impurity defect (b) Vacancies (i) Frenkel defect (ii) Schottky defect (ii) Line defects (one dimensional) (a) Edge dislocation (b) Screw dislocation (iii) Surface defects (two dimensional) (a) Grain boundaries (b) Twin boundaries (c) Tilt boundaries (d) Stacking faults (e) Ferromagnetic domain walls (iv) Volume defects (three dimensional) (a) Cavities or voids (b) Cracks and holes 14. What is impurity defect? What are types of impurity defects? A foreign substance added to a crystal is called impurity. The impurity atom may fit in the structure in two ways giving rise to two kinds of impurity defects. (i) Substitution impurity defect (ii) Interstitial impurity defect 15. What are vacancies? Vacancies are empty atomic sites. Vacancies may occur as a result of imperfect packing during the original crystallization or they may arise from the thermal vibrations of atoms at higher temperatures. There are different kinds of vacancies like Frenkel defect, Schottky defect, Colour centers etc. 16. What is Frenkel defect? A vacancy associated with interstitial impurity is called Frenkel defect. 17. What is Schottky defect? If an atom is missing from its lattice site, the vacancy is called Schottky defect. 18. What is line defect? What are its type? The defect along a line is called line defect. There are two types of line defects (i) Edge dislocation and (ii) Screw dislocation. 19. What is burger's vector? The magnitude and the direction of the displacement due to edge dislocation are defined by a vector called Burger's vector. 20. What are twin boundaries? If the atomic arrangement on one side of the boundary is the mirror image of the arrangement on the other side the defect is called twin boundaries. 21. What is stacking fault? This defect arises due to defect in the stacking of atomic planes. In some cases a part of certain atomic plane will be missing where as in some other cases a portion of extra atomic plane is present, changing the sequence of arrangement of atoms.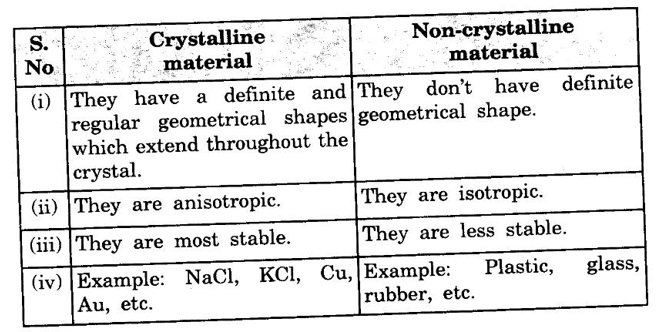
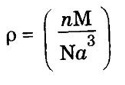
Materials Science: Unit I: Crystallography : Tag: : Crystallography | Materials Science - Additional 2 Marks Question & Answers
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
