Materials Science: Unit I: Crystallography
2 Marks Question & Answers
Crystallography | Materials Science
2 Marks Question & Answers : Crystallography - Materials Science
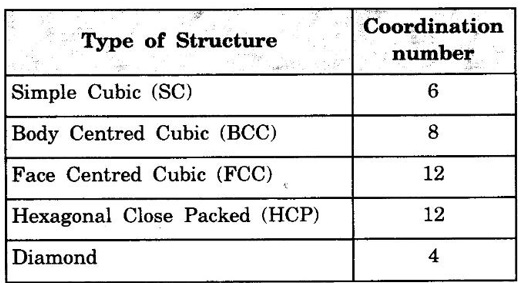
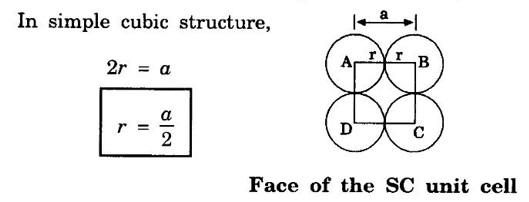
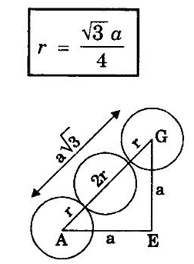
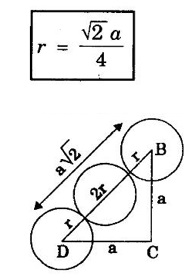
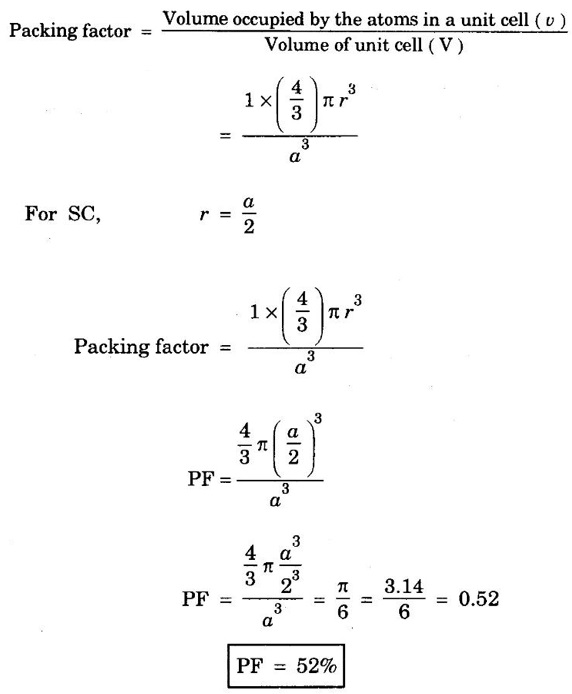
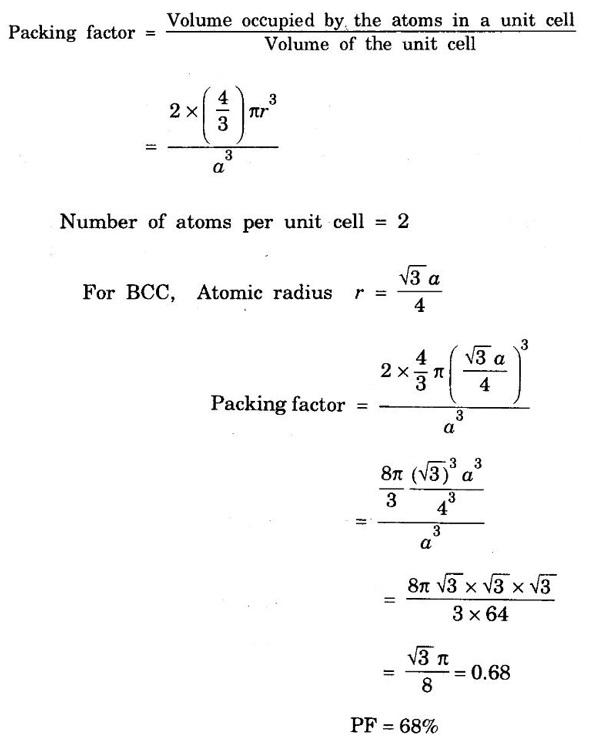
ANNA UNIVERSITY - PART - A '2' Marks Q & A 1. What is a space lattice? It is an array of points in three dimensions in which every point has an identical surroundings. 2. What is a unit cell? It is the smallest volume of a solid from which the entire crystal structure can be constructed by repetition in three-dimension. 3. Name the seven crystal systems. (i) Cubic (ii) Tetragonal (iii) Orthorhombic (iv) Monoclinic (v) Triclinic (vi) Rhombohedral (vii) Hexagonal 4. What is primitive cell? A primitive cell is the simplest type of unit cell which contains only one lattice point per unit cell. 5. Name the crystal structure of the following: (a) Gold (b) Germanium (c) Barium (d) Zinc (a) Gold FCC (b) Germanium - Diamond cubic (c) Barium - BCC (d) Zinc – HCP 6. Bismuth has a = b = c = 4.74 Å and angles α = β = γ = 60°. What is its crystal structure? Given a = b = c = 4.74 Å, α = β = γ = 60° Since a = b = c and α = β = γ ≠ 90° The crystal structure of Bismuth is trigonal (Rhombohedral). 7. What are Bravais lattices? There are only 14 ways of arranging points in 3 - dimensional space such that the environment looks same from each point. ie., there are 14 possible types of space lattices out of the seven crystal systems. These 14 space lattices are called Bravais lattices. 8. Give the values of number of atoms in unit cell for SC, BCC, FCC and HCP. 9. Define coordination number. It is the number of nearest neighbouring atoms that any atom has in the given crystal structure. 10. Give the coordination numbers for SC, BCC, FCC, HCP and Diamond 11. Define atomic radius. The half of the distance between nearest neighbouring atoms in a crystal is known as atomic radius. The atomic radius is denoted by 'r' and it is usually expressed in terms of the cube edge 'a' (lattice parameter). 12. Obtain the formula for atomic radius 'r' in terms of lattice constant 'a' for simple cubic. 13. Arrive at an expression for atomic radius in terms of lattice constant for BCC. In BCC, along the body diagonal AG, r + 2r + r = a √3 4r = a √3 14. Derive an expression for atomic radius in terms of lattice parameter for FCC. For FCC, along any face diagonal, r + 2r + r = a √2 4r = a √2 15. Define packing factor. What is its unit? It is the ratio of volume of atoms in unit cell to the volume of the unit cell. It has no unit, since it is a ratio of same physical quantity. 16. Calculate packing factor in the case of simple cubic structure. 17. Calculate packing factor of body centred cubic crystal.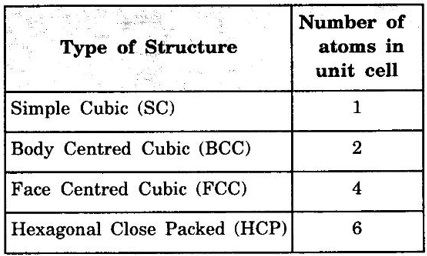
Materials Science: Unit I: Crystallography : Tag: : Crystallography | Materials Science - 2 Marks Question & Answers
Related Topics
Related Subjects
Materials Science
PH3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
