Basic Electrical and Electronics Engineering: Unit III: Analog Electronics
Types of Semiconductors
A pure form of Semiconductor is known as intrinsic semiconductor.
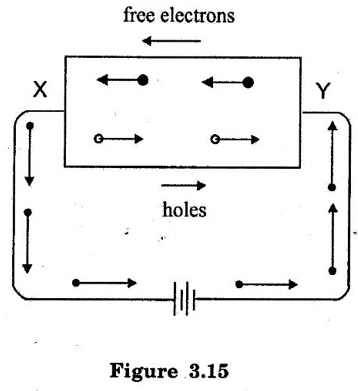
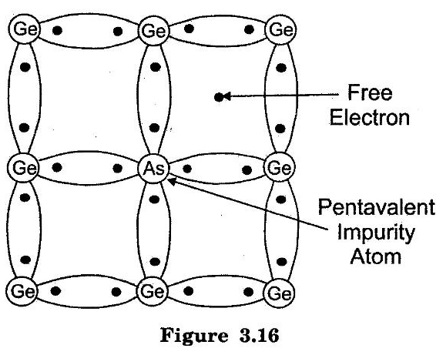
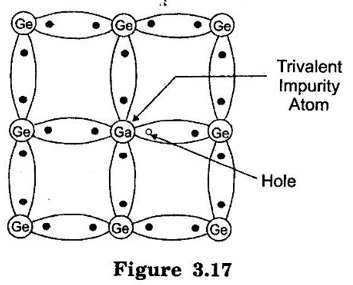
TYPES OF SEMICONDUCTORS Semiconductor may be classified as under: A pure form of Semiconductor is known as intrinsic semiconductor. Even at room temperature some of the valence electrons acquire sufficient energy to enter the conduction band to form free electrons. When the electric field is applied, the current conduction takes place by free electrons and holes. A missing electron in the valence band leaves a vacant space which is known as a hole. (refer figure 3.14.) Holes also contribute to current. Therefore the total current inside the semiconductor is sum of currents due to free electrons and holes. It may be noted that current in the external wire is fully by electrons. In figure 3.15 holes being positively charged move towards negative terminal of the supply. As the holes reach the negative terminal Y, electrons enter the semiconductor near the terminal and combine with the holes. Simultaneously, the loosely held electrons near the terminal X are attracted away from their atoms into the positive terminal. This creates new holes near the positive terminal which inturn float towards the negative terminal due to thermal agitation. At room temperature, intrinsic semiconductor has poor conduction. So it is not useful in electronic devices. Where as an extrinsic semiconductor is an improved intrinsic semiconductor with a small amount of impurities added by a process, known as doping, which alters the electrical properties of the semiconductor and improves its conductivity. Adding impurities into the semiconductor materials (doping process) can control their conductivity. It is called impurity or extrinsic semiconductor. A pentavalent impurity containing five valence electrons then it is called as donor impurity. A pentavalent impurity is added to the semiconductor, a large number of free electrons are produced in the semiconductor. Examples of pentavalent impurities are Arsenic, Phosphorous and Antimony. A trivalent impurity containing three valence electrons then it is called as acceptor impurity. A trivalent impurity creates a large number of holes in the semiconductor crystal. Examples of trivalent impurities are Boron, Aluminium and Indium. Depending upon the type of impurity added, extrinsic semiconductors are classified into two types. They are: (i) n-type semiconductor (ii) p-type semiconductor 1. n-type Semiconductor When a small amount of pentavalent impurities such as Arsenic, Phosphorous or Antimony is added to a intrinsic semiconductor, it is known as n-type semiconductor. Atomic number for arsenic (As), antimony (Sb) are 33 and 51 respectively. Pentavalent impurities are called as donor impurities because they donate or provide free electrons to the semiconductor crystal. In n-type semiconductor, net concentration of electrons greater than that of holes. In figure 3.16, an Arsenic impurity (pentavalent impurity) which is surrounded by germanium atoms. The germanium atom has five valence electrons. Four out of these five electrons form the covalent bonds with four germanium atoms. The fifth valence electron of arsenic atom finds no chance of forming covalent bond and is thus free as shown in figure. 3.16. This electron is loosely bound to its arsenic atom. A very small amount of energy is required to de-attach this electron from nucleus of its arsenic atom. Every arsenic atom donates one electron to the conduction band. Hence the pentavalent impurities are called as donor impurities. On giving electrons for conduction, the donor atom becomes positively charged ion because this is held tightly by covalent bonds in crystal. This unable to move and hence it is called as positively charged immobile ion. But it cannot take part in conduction because it is firmly stable in the crystal. The addition of pentavalent impurity to the semiconductor produces more number of free electrons in the semiconductor and the conductivity of the N-type semiconductor increases. As a result of doping, net concentration of electrons greater than that of holes. Hence, in N-type semiconductor electrons are majority carriers and holes are minority carriers. 2. p-type Semiconductor When a small amount of trivalent impurities such as Boron, Aluminium, gallium or Indium is added to a intrinsic semiconductor, it is known as p-type semiconductor. Atomic number for boron, gallium and indium are 5, 31 and 49 respectively. The addition of trivalent impurity to the semiconductor produces more number of holes in the semiconductor. Trivalent impurities are known as acceptor impurities because the holes created can accept the electrons. Consider a small amount of trivalent impurity like gallium is added to intrinsic germanium. Since small amount of impurity, it may be assumed that each impurity is surrounded by germanium atoms. In figure 3.17, Gallium is trivalent impurity and it has three valence electrons. These three electrons form the covalent bonds with three neighbouring germanium atom. The fourth germanium atom cannot make covalent bond with gallium. It is because gallium does not have fourth valence electron as shown in Figure 3.17. Hence fourth covalent bond is incomplete. The fourth bond is incomplete; because there is a shortage of one electron. This missing electron is called a hole. A vacancy or hole having tendency to complete the covalent bond from neighbouring atoms. An electron from neighbouring atoms requires some energy to jump into the vacancy. Thus, for each gallium atom added, one hole is created. A small amount of gallium provides large number of holes. The addition of trivalent impurity(gallium) to the intrinsic semiconductor produced a large number of holes. Trivalent impurity are called as acceptor impurity. It is because it accepts free electrons in the place of holes. Each gallium atom donates a hole for conduction, it becomes negatively charged ion. In p- type semiconductor, net concentration of holes greater than that of electrons hence, holes are majority carriers and electrons are minority carriers.
1. Intrinsic Semiconductors:

2. Extrinsic Semiconductor
3. Comparison of Intrinsic and Extrinsic Semiconductors
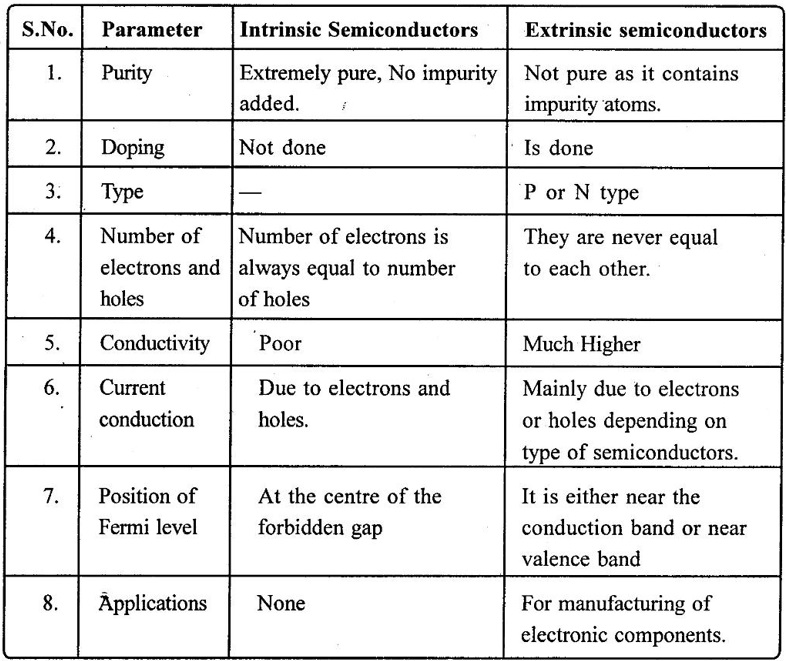
4. Comparison of P-type and N-Type semiconductors

Basic Electrical and Electronics Engineering: Unit III: Analog Electronics : Tag: : - Types of Semiconductors
Related Topics
Related Subjects
Basic Electrical and Electronics Engineering
BE3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
Basic Electrical and Electronics Engineering
BE3251 2nd Semester CSE Dept 2021 | Regulation | 2nd Semester CSE Dept 2021 Regulation

