Basic Electrical and Electronics Engineering: Unit III: Analog Electronics
Semiconductor
Properties, Bonds, Commonly Used Semiconductors, Energy Band Description
A semiconductor material is one whose electrical properties lie in between those of insulators (glass) and good conductors (copper).
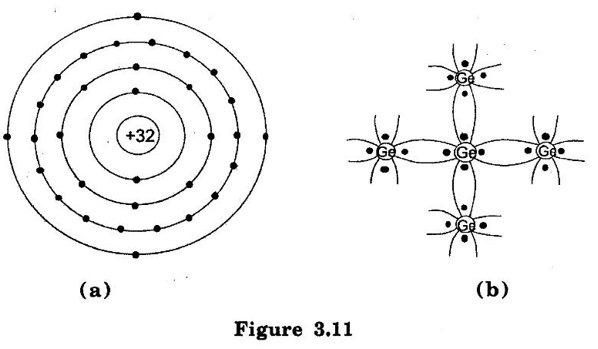
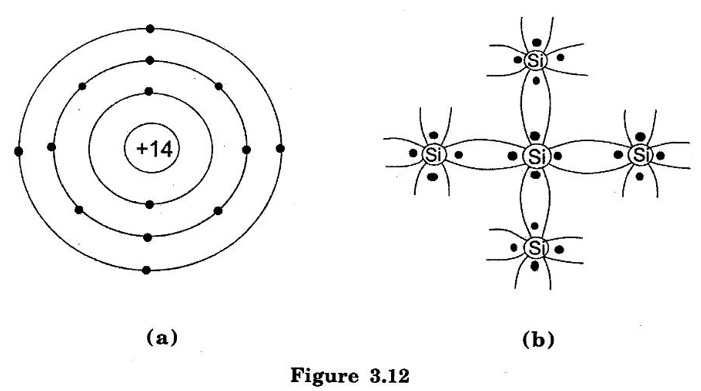
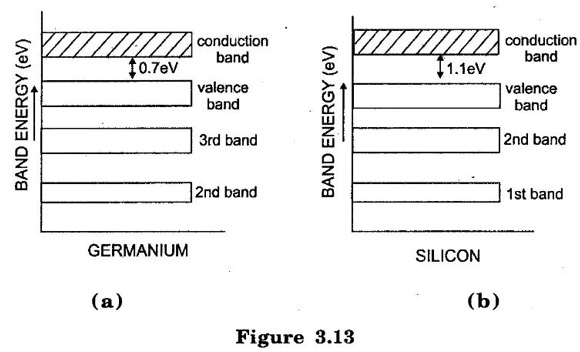
SEMICONDUCTOR ● A semiconductor material is one whose electrical properties lie in between those of insulators (glass) and good conductors (copper). Examples are: germanium, silicon, selenium and carbon etc. ● Transistors are only one of the family of semiconductor devices. Generally, a semiconductor is defined on the basis of electrical conductivity as under: ● "A semiconductor is a substance which has resistivity (10-4 to 0.5Ωm) in between conductors and insulators e.g. germanium, silicon, selenium, carbon etc." (i) The resistivity of a semiconductor is less than an insulator but more than a conductor. (ii) Semiconductors have negative temperature co-efficient of resistance. The conductivity of the semiconductor is increases with increase in temperature. This means the resistance of a semiconductor decreases with the increase in temperature For example, at low temperatures germanium is behaves an insulator but it becomes a good conductor at high temperatures. (iii) By adding some amount of impurity atoms (e.g. arsenic, gallium etc.) to a semiconductor, its current conductivity properties change appreciably. Semiconductors are made up of individual atoms bonded together in a regular, periodic structure to form an arrangement whereby each atom is surrounded by 8 electrons. The bonding action of valence electrons is due to the fact that it is the tendency of each atom to complete its last orbit by acquiring 8 electrons in it. In the formation of a co-valent bond, each atom contributes equal number of valence electrons and the contributed electrons are shared by the atoms engaged in the formation of the bond. Figure. 3.10 shows the co-valent bonds over germanium atoms. A germanium atom has four valence electrons. Each germanium atom to have eight electrons in the last orbit. To form the co-valent bond, each germanium atom positions itself between four other germanium atoms as shown in Figure.3.10(a). Each neighbouring atom shares one valence electron with the central atom. In this business of sharing, the central atom completes its last orbit by having 8 electrons revolving around the nucleus. In this way, the central atom sets up co-valent bonds. Figure 3.10(b) shows the bonding diagram. The following points may be noted regarding the co-valent bonds : i) Co-valent bonds are formed by sharing of valence electrons. ii) In the formation of co-valent bond, each valence electron of atom forms direct bond with the valence electron of adjacent atom. In other words, valence electrons are associated with particular atoms. For this reason, valence electrons in a semiconductor are not free. Germanium (Ge) and silicon (Si) are Commonly used Semiconductors. The energy required to break the co-valent bonds of these semiconductors (i.e. energy required to release an electron from their valence bands) is very small; being about 0.7 eV for germanium(Ge) and about 1.1 eV for silicon (Si). (i) Germanium The atomic number of germanium is 32. Therefore, it has 32 protons and 32 electrons. The first orbit accommodates two electrons. The second orbit accommodates eight electrons, third orbit accommodates eighteen electrons and outer or valence orbit accommodates four electrons [See Figure. 3.11 (a)]. It is clear that germanium atom has four valence electrons i.e., it is a tetravalent element. Figure. 3.11(b) shows how the various germanium atoms are held through covalent bonds. Since the atoms are arranged in an orderly pattern, Hence, germanium has crystalline structure. (ii) Silicon Silicon is an element in most of the common rocks. In fact, sand is silicon dioxide. The silicon compounds are chemically reduced to silicon which is 100% pure for use as a semiconductor. The atomic number of silicon is 14. Therefore, it has 14 protons and 14 electrons. The first orbit accommodates two electrons. The second orbit accommodates eight electrons, third orbit accommodates four electrons [See Figure 3.12(a)]. It is clear that silicon atom has four valence electrons i.e. it is a tetravalent element. Figure 3.12(b) shows how various silicon atoms are held through co-valent bonds. Similar to germanium, silicon atoms are also arranged in an orderly manner. Hence, silicon has crystalline structure. We know that a semiconductor is a substance whose resistivity lies between conductors and insulators. There is an energy gap EG between the valence band and nearly empty conduction band, it is called forbidden energy gap. Figure 3.13(a) and 3.13(b) shows the energy band diagrams of germanium and silicon respectively. It may be seen that forbidden energy gap is very small; being 1.12 eV for silicon and 0.72 eV for germanium. If we provide some energy to silicon, electrons will be lifted from valence band to conduction band. Hence, at room temperature, a piece of germanium or silicon is neither a good conductor nor an insulator. Due to this reason, such substances are called semiconductors.Properties of Semiconductors
Bonds in Semiconductors

Commonly Used Semiconductors
Energy Band Description of Semiconductors
Basic Electrical and Electronics Engineering: Unit III: Analog Electronics : Tag: : Properties, Bonds, Commonly Used Semiconductors, Energy Band Description - Semiconductor
Related Topics
Related Subjects
Basic Electrical and Electronics Engineering
BE3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
Basic Electrical and Electronics Engineering
BE3251 2nd Semester CSE Dept 2021 | Regulation | 2nd Semester CSE Dept 2021 Regulation

