Basic Electrical and Electronics Engineering: Unit III: Analog Electronics
Insulators, Metals and Semiconductors on the basis of Band Gap
"Insulators are those substances which do not allow electric current through them. Examples, Rubber, Wood, Glass etc."
INSULATORS, METALS AND SEMICONDUCTORS ON THE BASIS OF BAND GAP "Insulators are those substances which do not allow electric current through them. Examples, Rubber, Wood, Glass etc." The forbidden energy gap is very large and is greater than 5 eV. In insulators, Valence band is completely filled and the conduction band is empty. The large electric field is required to lift the electrons from valence band to conduction band. At room temperature, the valence electrons of an insulator can not have so much energy to cross over to the conduction band. Therefore as temperature increases, the resistance of insulator decreases. Insulator has negative temperature coefficient of resistance. "Conductors are substances which allow the flow of electric current through them. Examples: Copper, Aluminium, Salt solutions etc.," In conductors the valence band and conduction band overlap each other, as shown in figure 3.8 (b). Because of this overlapping, a small potential difference across a conductor causes the free electrons to constitute the current. A metal consists of a large number of free electrons without giving any external energy. Hence a metal work as a very good conductor. "Semiconductors are those substances which allow electric charges partially to flow through them. Examples: Germanium, Silicon etc.," In semiconductors, the forbidden energy gap is not very wide. It is 0.72 eV for germanium and 3.12 eV for silicon. In semiconductors, valence band is almost filled and conduction band is almost empty. A small electric field is required to lift the electrons from valence band to conduction band. At low temperature, the valence band is completely filled and conduction band is completely empty. Hence at low temperature semiconductors act as insulators. At room temperature, some electrons lifted to conduction band from valence band. So at room temperature semiconductors are able to conduct some current. If the temperature is further increased above room temperature, more valence electrons cross over to the conduction band. Hence semiconductor have negative temperature coefficient of resistance. The valence electrons in germanium are in the fourth shell/orbit while the valence electrons in silicon are in the third shell/orbit. i.e., closure to nucleus. It means that the germanium valence electrons are at higher energy level than those in silicon. So the germanium valence electrons will need smaller amount of additional energy to escape from the atom. Hence germanium produces more number of electron hole pairs than silicon. Therefore the leakage current is more in germanium than silicon. This property makes germanium more unstable at high temperatures. Therefore silicon is most widely used in semiconductors than germanium.(i) Insulators:
(ii) Conductors:
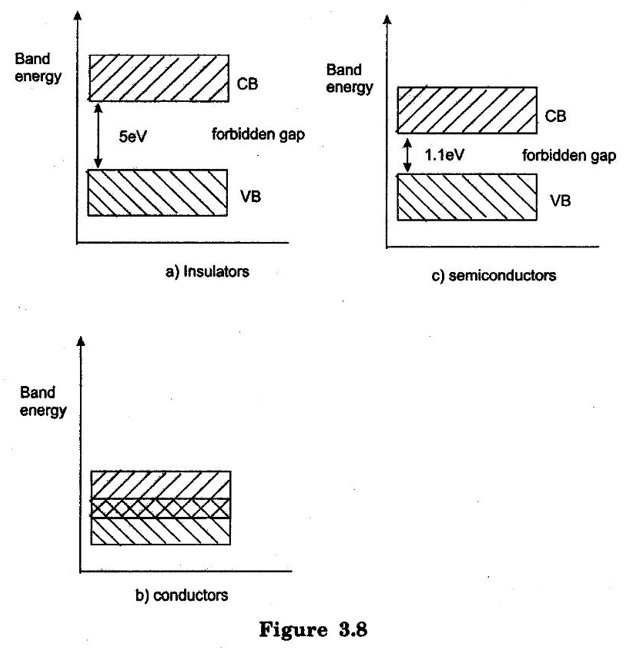
(iii) Semiconductors:
Why silicon is most widely used in Semiconductor devices?

Basic Electrical and Electronics Engineering: Unit III: Analog Electronics : Tag: : - Insulators, Metals and Semiconductors on the basis of Band Gap
Related Topics
Related Subjects
Basic Electrical and Electronics Engineering
BE3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
Basic Electrical and Electronics Engineering
BE3251 2nd Semester CSE Dept 2021 | Regulation | 2nd Semester CSE Dept 2021 Regulation

