Basic Electrical and Electronics Engineering: Unit III: Analog Electronics
Important Energy Bands in Solids
As discussed earlier, individual X, Y, Z etc. energy levels of an isolated atom are converted as energy bands when the atom is in a solid.
IMPORTANT ENERGY BANDS IN SOLIDS As discussed earlier, individual X, Y, Z etc. energy levels of an isolated atom are converted as energy bands when the atom is in a solid. Though there are a number of energy bands in solids, the following are of particular importance: "The energy band which possesses valence electrons is known as valence band". The electrons in the outermost orbit of an atom are known as valence electrons. The energy band possesses the valence electrons is called valence band. In a normal atom, valence band may be completely or partially filled. "The energy band which possesses free electrons is known as conduction band". Electron in this band is responsible for conduction. If a substance has empty conduction band, it means current conduction is not possible in that substance. Generally, insulators have empty conduction band. If we provide some energy to the atom, electrons move from valence band to conduction band. "There is a energy gap between conduction band and valence band on the energy level diagram is known as forbidden energy gap." Forbidden energy gap is a region where no electron can stay as there is no allowed energy state in this region. The width of the forbidden energy gap is a measure of the bondage of valence electrons to the atom. If the energy gap is greater, the valence electrons are more tightly bound to the nucleus. To lift an electron from valence band to the conduction band (i.e. to make the valence electron free), external energy through heat, light equal to the forbidden energy gap should be supplied.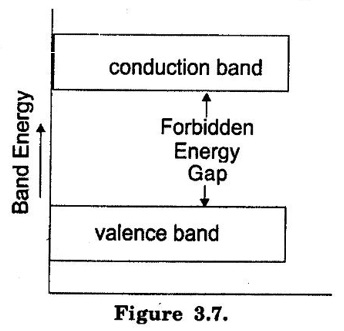
(i) Valence band.
(ii) Conduction band.
(iii) Forbidden energy gap.
Basic Electrical and Electronics Engineering: Unit III: Analog Electronics : Tag: : - Important Energy Bands in Solids
Related Topics
Related Subjects
Basic Electrical and Electronics Engineering
BE3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
Basic Electrical and Electronics Engineering
BE3251 2nd Semester CSE Dept 2021 | Regulation | 2nd Semester CSE Dept 2021 Regulation

