Basic Electrical and Electronics Engineering: Unit III: Analog Electronics
Bohr's Atom Model
In 1913, Neils Bohr, Danish Physicist gave clear explanation about atomic structure. According to Bohr atom model:
BOHR'S ATOM MODEL In 1913, Neils Bohr, Danish Physicist gave clear explanation about atomic structure. According to Bohr atom model: (i) An atom consists of negatively charged electrons which rotate around positively charged nucleus. (ii) The electrons can revolve only in permitted orbit and not in any intermediate orbit. (iii) The electrons in each permitted orbit have fixed amount of energy. The electrons in the outer orbit have more energy than the electron in the inner orbit. (iv) If an electron is given additional energy by means of heat, light and etc., it is shifted to the higher orbit. The atom is said to be in a state of excitation. But this state will not be static, because the electron soon returns back to the original lower orbit. As it return to the original state, it gives back the acquired energy in the form of heat, light or other radiations. Fig. 3.4 shows the structure of silicon atom. It has 14 electrons. Two electrons revolve in the first inner orbit, eight electrons in the second orbit and 4 electrons in the third or outer orbit. The first, second, third orbits are also identified as X, Y, Z orbits respectively. These electrons can revolve only in permitted orbits and not in any intermediate orbit. Therefore, all radii between r1 and r2 or between r2 and r3 are forbidden.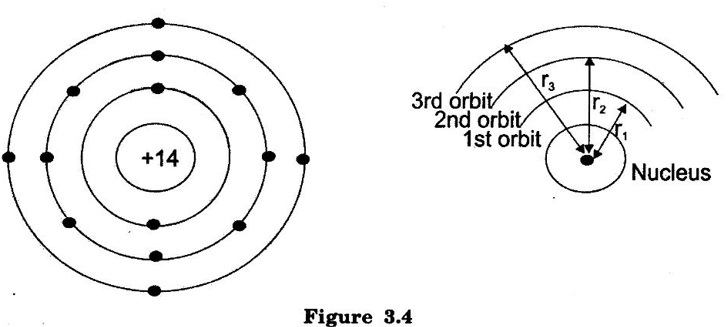
Basic Electrical and Electronics Engineering: Unit III: Analog Electronics : Tag: : - Bohr's Atom Model
Related Topics
Related Subjects
Basic Electrical and Electronics Engineering
BE3251 2nd semester Mechanical Dept | 2021 Regulation | 2nd Semester Mechanical Dept 2021 Regulation
Basic Electrical and Electronics Engineering
BE3251 2nd Semester CSE Dept 2021 | Regulation | 2nd Semester CSE Dept 2021 Regulation

