Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics
Simple crystal structures
It has been found that most of the common metals possess cubic or hexagonal structures only.
SIMPLE CRYSTAL STRUCTURES It has been found that most of the common metals possess cubic or hexagonal structures only. The important simple crystal structures are: 1. Simple Cubic (SC) structure, 2. Body Centred Cubic (BCC) structure, 3. Face Centred Cubic (FCC) structure, and 4. Hexagonal Close Packed (HCP) structure. ✓ The unit cell of simple cubic structure has one atom at each corner. So there are eight corner atoms. ✓ The arrangement of lattice points in a SC cell is shown in Figs.0.6 (a) and (b). ✓ The unit cell of BCC has atom at each corner. Also it has one atom at the centre of the body. So we have eight corner atoms and one centre atom. ✓ The arrangement of lattice points in a BCC cell is shown in lano Figs.0.7 (a) and (b). ✓ Examples: Tungsten, vanadium, molybdenum, chromium, alkali metals, α-iron (below 910°C), δ-iron (1400°C to 1539°C) etc. ✓ The unit cell of FCC has one atom at each corner. It also has one atom at the centre of the each face. So it has eight corner atoms and six face centred atoms. ✓ The arrangement of lattice points in a FCC cell is shown in Figs.0.8 (a) and (b). ✓ Examples: Copper, silver, gold, aluminium, nickel, lead, platinum, γ-iron (910°C to 1400°C), etc. ✓ The unit cell HCP contains one atom at each corner of the hexagonal prism, one atom each at the centre of the hexagonal faces and three more atoms within the body of the cell. ✓ The arrangement of lattice points in a HCP cell is shown in Figs.0.9 (a), (b) and (c). ✓ Examples: Magnesium, zinc, titanium, zirconium, beryllium, cadmium, etc.1. Simple Cubic (SC) structure
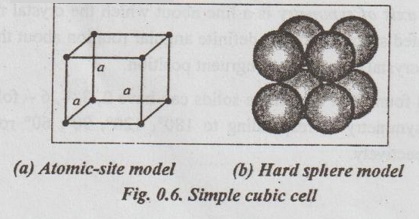
2. Body Centre Cubic (BCC) structure
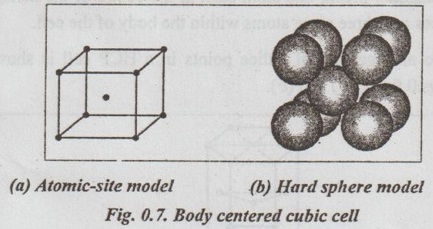
3. Face Centred Cubic (FCC) structure
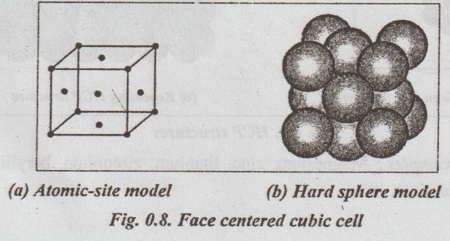
4. Hexagonal Close Packed (HCP) structure
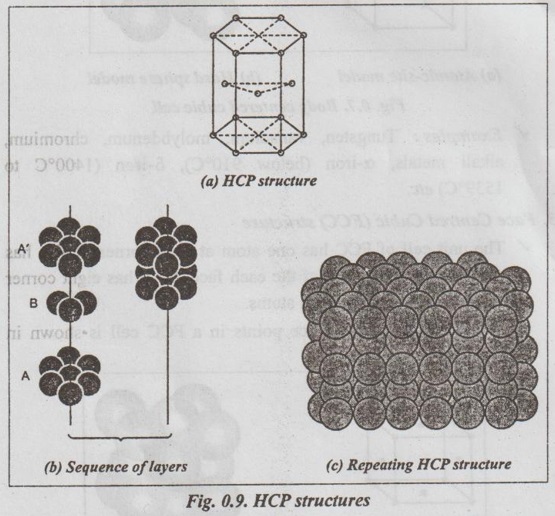
Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics : Tag: : - Simple crystal structures
Related Topics
Related Subjects
Engineering Materials and Metallurgy
ME3392 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
