Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics
Crystallographic Terms
The various terms associated with crystallography are:
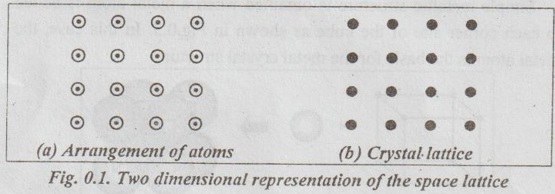
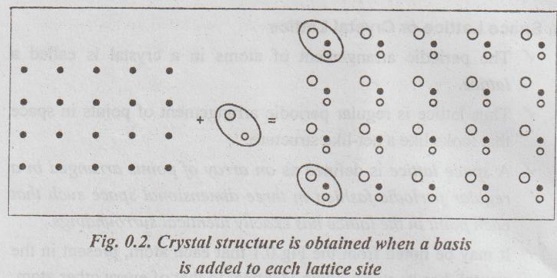
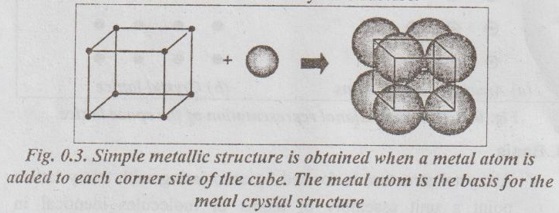
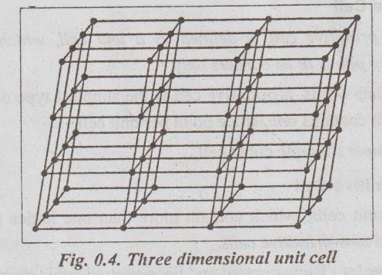
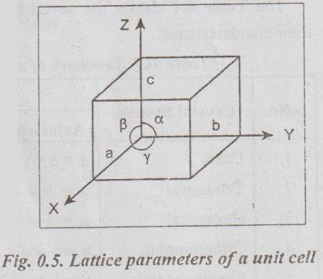
CRYSTALLOGRAPHIC TERMS The various terms associated with crystallography are: 1. Crystal: A crystal is a solid whose constituent atoms or molecules are arranged in a systematic geometric pattern. 2. Lattice Points: Lattice points denote the position of atoms or molecules in the crystals. ✓ The periodic arrangement of atoms in a crystal is called a lattice. ✓ Thus lattice is regular periodic arrangement of points in space that looks like a net-like structure. ✓ A space lattice is defined as an array of points arranged in a regular periodic fashion in three dimensional space such that each point in the lattice has exactly identical surroundings. ✓ It may be noted from the Fig.0.1 that each atom, present in the crystal, has its surrounding identical to that of every other atom, in the two dimensional lattice. ✓ A crystal structure is formed by associating with every lattice point a unit assembly of atoms or molecules identical in composition. This unit assembly is called basis. ✓ The basis must be identical in composition, arrangement and orientation such that the crystal appears exactly the same at one point as it does at other equivalent points. ✓ The basis repeated with correct periodicity in all directions gives the actual crystal structure. ✓ Fig.0.2 shows the basis consisting of a group of two atoms. When the basis is associated with each lattice site, the crystal structure is obtained. ⸫ Space lattice + Basis → Crystal structure Illustration: Simple metallic structure is obtained when a metal atom is added to each corner site of the cube as shown in Fig.0.3. In this case, the metal atom is the basis for the metal crystal structure. Fig. 0.3. Simple metallic structure is obtained when a metal atom is added to each corner site of the cube. The metal atom is the basis for the metal crystal structure ✔ The atoms in a crystal are arranged in a periodic array. It is possible to isolate a group of atoms or molecules or ions which represents all the characteristics of the crystal. This representative unit is called unit cell. ✔ Unit cell is defined as the fundamental elementary pattern of minimum number of atoms or molecules or group of molecules which represent fully all the characteristics of the crystal. ✔ It is also defined as the smallest portion of the space lattice, the repetition of which in three dimension (Fig.0.4) will give the actual crystal structure. ✔A unit cell is also known as basic cell or basic unit or fundamental unit or fundamental elementary pattern or building block or lattice unit. ✔ Importance of the Unit Cell: We can analyse the crystal as a whole by investigating a combination of various unit cells. ✓ A unit cell is completely defined by the length of its edges (i.e., the distance between two neighbouring points along the three dimensions) and the angle between them. ✓ Fig.0.5 shows a unit cell of a three dimensional crystal lattice. It is formed by intercepts a, b and c along the three axes. The three angles α, β and γ (ie., angle between the intercepts) are called interfacial angles. ✓ Both the intercepts a, b, c and α, β, γ interfacial angles constitute the lattice parameters of the unit cell. ✓ If the values of intercepts and interfacial angles are known, we can easily determine the form and actual size of the unit cell. ✓ The primitive cell is defined as a unit cell, which contains lattice points at its corners only: ✓ In other words, a primitive cell is the simplest type of unit cell which contains one lattice point per unit cell. ✓ Example: Simple cubic cell. ✓ The unit cells, which contain more than one lattice point, are called non-primitive cells. ✓ Examples: Body-centred and Face-centred cubic structures..3. Space Lattice or Crystal Lattice
4. Basis
5. Unit Cell
6. Lattice Parameters of a Unit Cell
7. Primitive Cell
8. Non-primitive Cell
Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics : Tag: : - Crystallographic Terms
Related Topics
Related Subjects
Engineering Materials and Metallurgy
ME3392 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
