Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics
Coordination Number
Definition, Calculation | Crystal Physics
The coordination number is defined as the number of nearest atoms which are directly surrounding a given atom.
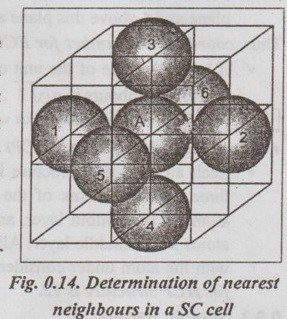
Coordination Number ✓ The coordination number is defined as the number of nearest atoms which are directly surrounding a given atom. ✓ It may also be defined as the nearest neighbours to an atom or an ion in a crystal. ✓ Coordination number is used for analysing the bonding of atoms within materials. ✓ The concept of coordination number is very important for making study of the crystal structure of solids. ✓ The coordination number of a given crystal structure is an indication of the closeness of the packing of atoms. ✓ When the coordination number is larger, the structure is more closely packed. ✓ Coordination number can be calculated as follows in various crystal structures. ✓ The unit cell of simple cubic lattice has one atom at each of the eight corners. It is observed that a corner atom is surrounded by six equidistant neighbours, as shown in Fig.0.14. Hence the coordination number of simple cubic lattice is six. ✓ If 'a' is the side of the unit cell, then the distance between the nearest neighbours will be 'a'. ✓ The unit cell of body centred cubic lattice has one atom at each of the eight corners and one atom at the body centre. So it is obvious that the centre atom is surrounded by eight equidistant neighbours, as shown in Fig.0.15. Hence the coordination number of BCC structure is eight. ✓ If 'a' is the side of the unit cell then the distance between the nearest neighbours will be √3 a / 2 ✓ The unit cell of FCC structure has eight atoms at the eight corners and six atoms at the centre of the six faces. For any corner atom of the unit cell, the nearest atoms are the face centred atoms. ✓ For any corner atom, there are 4 face centred atoms in its own plane and 4 above this plane and 4 below this plane. Thus, coordination number for FCC structure = 4 + 4 + 4 = 12 ✓ If 'a' is the side of the unit cell then the distance between two nearest neighbours is a/√2. Note An FCC cell has the maximum value of the coordination number. ✓ Each atom in this structure is positional in valleys formed by three adjacent atoms of the top layer and by three adjacent atoms in the bottom layer, and is surrounded by six neighbour atoms in the middle layer. All these twelve atoms are in contact with the atom under consideration. Thus coordination number for HCP structure is 12.Calculation of coordination number
1. Simple Cubic (SC) structure
2. Body Centred Cubic (BCC) structure

3. Face Centre Cubic (FCC) structure
4. Hexagonal Close Packed (HCP) structure
Engineering Materials and Metallurgy: Unit 0: Review of Crystal Physics : Tag: : Definition, Calculation | Crystal Physics - Coordination Number
Related Topics
Related Subjects
Engineering Materials and Metallurgy
ME3392 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
