Engineering Thermodynamics: Unit I: Basics, Zeroth and First Law
Zeroth Law of Thermodynamics
Definition, Concept
Zeroth law of thermodynamics states, "When two bodies are in thermal equilibrium with a third body separately, they are also in thermal equilibrium with each other".
ZEROTH LAW OF THERMODYNAMICS Zeroth law of thermodynamics states, "When two bodies are in thermal equilibrium with a third body separately, they are also in thermal equilibrium with each other". As shown in Figure 1.28, let, a system X be in thermal equilibrium with another system Y. Also, let, another system Z is in thermal equilibrium with the system Y. Then, from Zeroth law of thermodynamics, the system X is in thermal equilibrium with the system Z. Hence, system X and system Z are at same temperatures.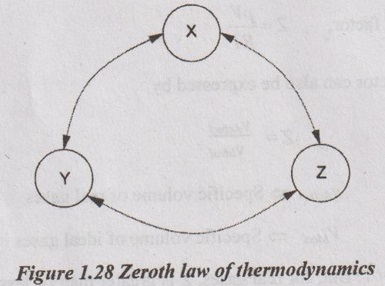
Engineering Thermodynamics: Unit I: Basics, Zeroth and First Law : Tag: : Definition, Concept - Zeroth Law of Thermodynamics
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
