Engineering Thermodynamics: Unit II: Second Law and Entropy
T-s Diagram
Entropy
A state of system is represented by selecting the properties i.e. temperature (T) and entropy (s) as co-ordinates as shown in Figure 2.33.
T-s DIAGRAM A state of system is represented by selecting the properties i.e. temperature (T) and entropy (s) as co-ordinates as shown in Figure 2.33. This diagram is known as T-s diagram. The differential form of heat transfer can be written as dQ = Tds When a system undergoes a change of states from state 1 to state 2, then the area under the process 1-2 represents the heat transfer from state 1 to state 2 as shown in Figure 2.33. Integrating above equation for state 1 to state 2, The quantity of heat transfer (Q1-2)rev is represented by the area under T-s diagram (i.e. area 1-2-s2-s1-1).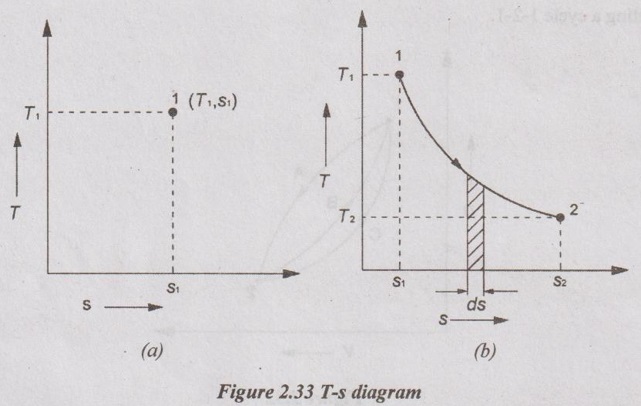

Engineering Thermodynamics: Unit II: Second Law and Entropy : Tag: : Entropy - T-s Diagram
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
