Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Steam Tables
Thermodynamics
In practice, it is tedious to calculate the relations between various quantities such as pressure, temperature, specific volume, enthalpy and entropy of steam at various stages.
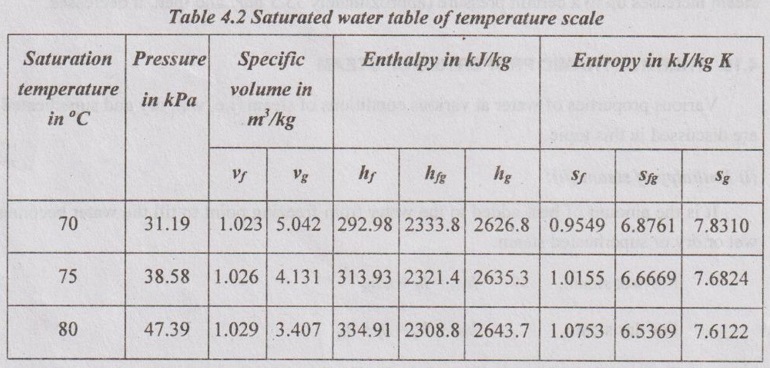
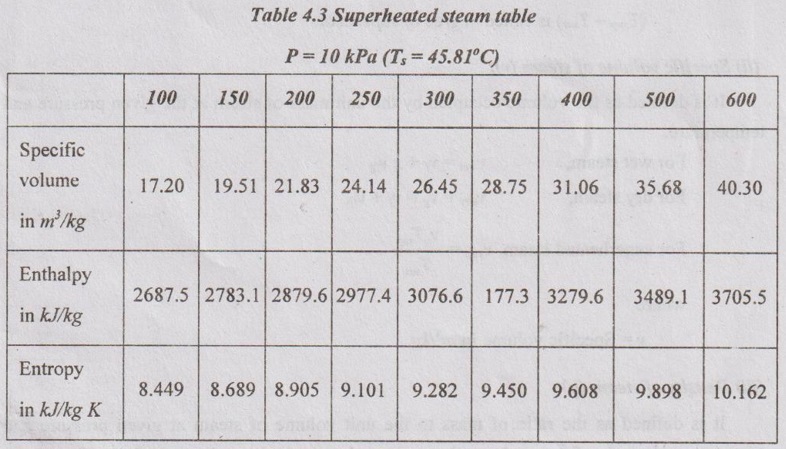
STEAM TABLES In practice, it is tedious to calculate the relations between various quantities such as pressure, temperature, specific volume, enthalpy and entropy of steam at various stages. These properties have experimentally been determined and tabulated in a specific manner. These tables are known as steam tables. The values of various quantities given in these tables are for 1 kg of steam which is dry saturated. The calculation for wet steam can easily be made by making a suitable use of such tables. The following three main divisions present in the steam tables. Table 4.1 shows the various quantities such as specific volume, specific enthalpy and specific entropy for various pressures. If the required pressure is not directly included in a steam table, it would be lying between two consecutive pressures. Such a data can be calculated by simple interpolation.. Table 4.2 shows the various quantities for various saturation temperatures since there is only one saturation temperature for each saturation pressure. Table 4.3 shows the superheated steam table. Here, the various quantities such as specific volume, specific enthalpy and specific entropy for various pressure and temperature are shown in a tabular form. From the properties of a saturated steam, the increase of pressure results in the gradual increase in sensible heat and decrease of latent heat of vaporization. But, the enthalpy of dry steam increases up to a certain pressure (approximately 33.5 bar) and then, it decreases.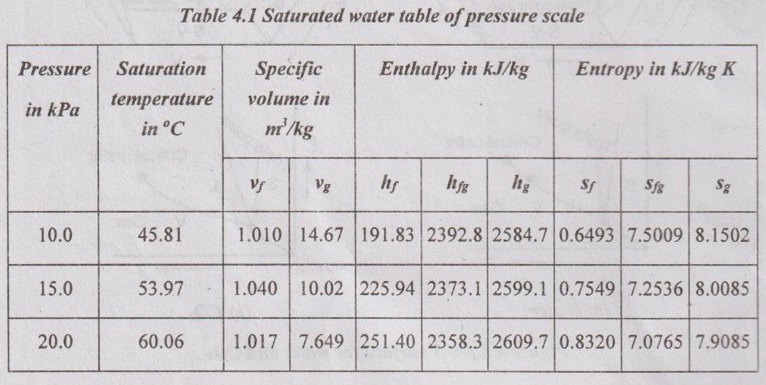
Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Thermodynamics - Steam Tables
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
