Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Solved Problems on Steam Properties
Thermodynamics
Solved Problems on Steam Properties: Properties of Pure Substances - Engineering Thermodynamics
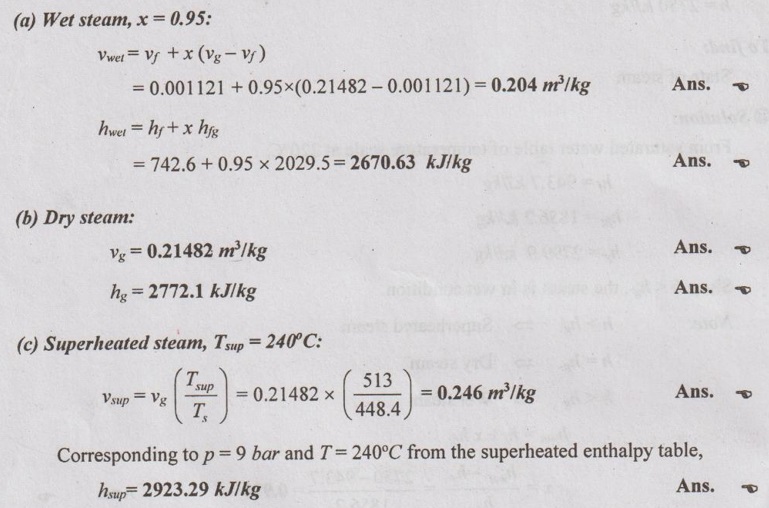
SOLVED PROBLEMS ON STEAM PROPERTIES Problem 4.1 Determine the state of steam at a pressure of 12 bar with its specific volume of 0.175 m3/kg. Given data: p = 12 bar v = 0.175 m3/kg To find: State of steam (whether dry, wet or superheated) Solution: From saturated water table of pressure scale at 12 bar, Specific volume of dry steam, vg = 0.16321 m3/kg, T, =188°C = 188 + 273 = 461 K Since v > vg, the steam is in superheated condition. Note: v > vg ⇒ Superheated steam v = vg ⇒ Dry steam v < vg ⇒ Wet steam Problem 4.2 Determine the condition of steam at a temperature of 220°C and enthalpy of 2750 kJ/kg. Given data: T = 220°C h = 2750 kJ/kg To find: State of steam Solution: From saturated water table of temperature scale at 220°C, hf = 943.7 kJ/kg hfg = 1856.2 kJ/kg hg = 2799.9 kJ/kg Since h < hg, the steam is in wet condition. Note: h > hg ⇒ Superheated steam h = hg ⇒ Dry steam h < hg ⇒ Wet steam Problem 4.3 Find the specific volume and enthalpy of steam at 9 bar when the condition of steam is (a) wet with dryness fraction 0.98 (b) dry saturated and (c) superheated and the temperature of steam 240°C. Given data: p = 9 bar (i) x = 0.95, (ii) x = 1, (iii) Superheated with Tsup = 240°C = 240 + 273 = 513 K Solution: From saturated water table of pressure scale at 9 bar, Ts = 175.4°C = 175.4 + 273 = 448.4 K vf = 0.001121 m3/kg vg = 0.21482 m3/kg hf = 742.6 kJ/kg hfg = 2029.5 kJ/kg hg = 2772.1 kJ/kg Problem 4.4 Find the internal energy of unit mass of steam at a pressure of 7 bar (i) when its quality is 0.8 (ii) when it is dry and saturated and (iii) superheated, the degree of superheat being 65°C. The specific heat of superheated steam at constant pressure is 2.277 kJ/kg K. Given data p = 7 bar = 700 kPa (i) x = 0.80 (ii) dry saturated and (iii) Superheated, (Tsup - Ts) = 65°C To find Internal energy Solution From saturated water table of pressure scale at 7 bar, T1 = 165°C, vf = 0.001108 m3/kg, vg = 0.27268 m3/kg hfg = 2064.9 kJ/kg hf = 697.1 kJ/kg, hg = 2762 kJ/kg, Sf = 1.992 kJ/kg K Sfg = 4.713 kJ/kg K Sg = 6.705 kJ/kg K Problem 4.5 Determine the condition of steam whether it is wet, dry or superheated for the following cases by using Steam tables only. (i) Steam has a pressure of 10 bar and specific volume 0.22 m3/kg. (ii) Steam has a pressure 15 bar and temperature 225°C. (iii) Steam has a temperature 200°C and enthalpy 2790.9 kJ/kg. (iv) Steam has a temperature of 120°C and entropy 7 kJ/kgK. Given data: (i) p = 10 bar and v = 0.22 m3/kg (ii) p = 15 bar and T = 225°C (iii) T = 200°C and h = 2790.9 kJ/kg (iv) T = 120°C and s = 7 kJ/kg K To find: Condition of steam Solution: (i) p = 10 bar and v = 0.22 m3/kg From saturated water table of pressure scale at 10 bar, vg = 0.1943 m3/kg Since v > vg, the steam is superheated. Ans. (ii) p = 15 bar and T = 225°C From saturated water table of pressure scale at 15 bar, Ts = 198.3°C Since T > Ts, the steam is superheated. Ans. (iii) T = 200°C and h = 2790.9 kJ/kg From saturated water table of temperature scale at 200°C, hg = 2790.9 kJ/kg Since h = hg, the steam is dry saturated. Ans. (iv) T = 120°C and s = 7 kJ/kgK From saturated water table of temperature scale at 120°C, sg = 7.129 kJ/kg K Since s < sg, the steam is in wet condition. Ans. Problem 4.6 One kg of steam contains 1/3 liquid and 2/3 vapour by volume. The temperature of the steam is 150°C. Find the quality, specific volume and specific enthalpy of mixture. Given data: m = 1 kg Vf = 1/3 Vg = 2/3 T3 = 150°C To find: Quality x, specific volume, v and specific enthalpy, h Solution: From saturated water table of temperature scale at 150°C,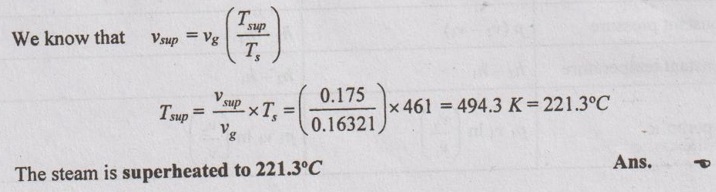
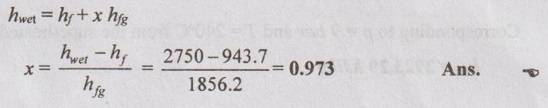






Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Thermodynamics - Solved Problems on Steam Properties
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
