Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Solved Problems on Non-Flow Processes
Thermodynamics
Solved Problems on Non-Flow Processes: Properties of Pure Substances - Engineering Thermodynamics
SOLVED PROBLEMS ON NON-FLOW PROCESSES Problem 4.7 Steam at 4 bar and 0.7 dry expands at constant volume until 5.5 bar. Find the final condition of steam and heat absorbed by 1 kg of steam. Given data: m = 1 kg p1 = 4 bar = 400 kPa x1 = 0.7 Process: Constant volume p2 = 5.5 bar = 550 kPa To find: Final condition of steam, x2 and Q Solution: From saturated water table of pressure scale at 4 bar, Ts = 143.6°C, hf1 = 604.7 kJ/kg hfg1 = 2132.9 kJ/kg, vf1 = 0.001084 m3/kg vg1 = 0.4622 m3/kg h1 = hf1 + x1hfg1 = 604.7 + 0.7 × 2132.9 = 2097.73 kJ/kg v1 = vf1 + x1(vg1 - vf1) = 0.001084 + 0.7 × (0.4622 - 0.001084) = 0.324 m3/kg From saturated water table of pressure scale at 5.5 bar, vf2 = 0.001097 m3/kg, hf2 = = 655.8 kJ/kg, vg2 = 0.3426 m3/kg, hfg2 = =2095.9 kJ/kg Volume is constant during the process. v2 = v1 = 0.324 m3 Since v2 < vg2, the steam is wet. v2 = vf2 + x2(vg2 - vf2) 0.324 = 0.001097 + x2 × (0.3426 - 0.001097) x2 = 0.946 dry Ans. h2 = hf2 + x2 hfg2 = 655.8 + 0.946 × 2095.9 = 2638.52 kJ/kg For constant volume process, W = 0 By first law of thermodynamics, Q = ΔU ⸫ Heat transfer, Q = U2 - U1 = (h2 - p2v2) - (h1 – p1v1) = (h2 – h1) - v2 (p2 - p1) [⸪ v1 = v2] = (2638.52 - 2097.73) - 0.324 (550 - 400) = 492.19 kJ Ans. Problem 4.8 A closed vessel of 0.2 m3 contains steam at 1 MPa and temperature 250°C. If the vessel is cooled so that pressure falls to 350 kPa. Determine the final temperature, heat transfer, and change of entropy during the process. Given data: V1 = 0.2 m3 p1 = 1 MPa = 10 bar = 1000 kPa T1 = 250°C p2 = 350 kPa = 3.5 bar To find: T2, Q and ΔS Solution: From saturated water table of pressure scale at 10 bar, Ts = 179.9°C, Since T1 > Ts, the state would be in the superheated region. From superheated specific volume table (in superheated region), At 10 bar and 250°C, v1 = 0.2328 m3/kg From saturated water table of pressure scale at 3.5 bar, vf2 = 0.001079 m3/kg vg2 = 0.52397 m3/kg hf2 = 584.3 kJ/kg hg2 = 2147.3 kJ/kg T2 = 138.9°C Since the vessel is closed, the cooling process is undergone by constant volume. For constant volume process, v2 = v1 = 0.2328 m3/kg The vapour must be wet in its final stage because vg2 > v2. v2 = vf2 + x2(vg2 - vf2) 0.2328 = 0.001079 + x2 × (0.52397 - 0.001079) x2 = 0.443 dry Ans. The final temperature of steam is equal to the saturation temperature at 3.5 bar. T2 = 138.9°C Final enthalpy of wet steam, h2 = hf2 + x2 hfg2 h2 = 584.3 + 0.443 × 2147.3 = 1535.6 kJ/kg From superheated steam table at 10 bar and 250°C, h1 = 2943 kJ/kg Mass of steam, Heat transfer, Q = m (h1 - h2) = 0.859 (2943 - 1535.6) = 1208.96 kJ Ans. From superheated entropy table at 10 bar and 250°C, S1 = 6.926 kJ/kg K From saturated water table of pressure scale at 3.5 bar, Sf2 = 1.727 kJ/kg K Sfg2 = 5.212 kJ/kg K Final entropy, S2 = Sf2 + xSfg2 = 1.727 + 0.443 × 5.212 = 4.036 kJ/kg K Change in entropy, Δs = m (s2 - s1) = 0.859 (4.036 - 6.926) = -1.941 kJ/K Ans. Problem 4.9 Steam is contained in a closed vessel of 30 litres capacity at a pressure of 10 bar with dryness fraction 0.95. Calculate the internal energy. Due to loss by radiation, the pressure of steam falls to 7 bar. Calculate the total loss of heat and final quality of steam. Given data: V1 = 30 lits = 30 / 1000 = 0.03 m3 [⸪ 1000 litres = 1 m3] p1 = 10 bar = 10 × 100 = 1000 kPa x1 = 0.95 p2 = 7 bar = 700 kPa To find: U1, Q and x2 Solution: From saturated water table of pressure scale, corresponding to 10 bar, hf1 = 762.6 kJ/kg, vf1 = 0.001127 m3/kg, hfg1 = 2013.6 kJ/kg, vg1 = 0.1943 m3/kg v1 = vf1 + x1(vg1 - vf1) = 0.001127 +0.95 (0.1943 - 0.001127) = 0.1846 m3/kg h1 = hf1 + x1 hfg1 = 762.6 + 0.95 × 2013.6 = 2661.02 kJ/kg Mass of steam, Internal energy, U1 = mu1 = m (h1 – p1v1) = 0.163 (2661.02 - 1000 × 0.1846) = 403.66 kJ Ans. For closed vessel, v1 = v2 = 0.1846 m3/kg From saturated water table of pressure scale, corresponding to 7 bar, vf2 = 0.001108 m3/kg, hf2 = 697.1 kJ/kg, vg2 = 0.27268 m3/kg hfg2 = 2064.9 kJ/kg Now, vg2 > v2, ⸫ the steam is in wet condition. v2 = vf2 + x2 vfg2 = vf2 + x2 (vg2 - vf2) 0.1846 = 0.001108 + x2 (0.27268 - 0.001108) x2 = 0.676 Ans. h2 = hf2 + x2 hfg2 = 697.1 + 0.676 × 2064.9 = 2092.97 kJ/kg For constant volume process, W = 0 By first law of thermodynamics, Q =ΔU ⸫ Heat transfer, Q = U2 - U1 = m [(h2 - p2v2) - (h1 – p1v1)] = m [(h2 – h1) - v2 (p2 - p1)] [⸪ v1 = v2] = 0.163 [(2092.97 - 2661.02) - 0.1846 (1000 - 700)] = -101.62 kJ Ans. Problem 4.10 Steam initially at 400 kPa and 0.6 dry is heated in a rigid vessel of 0.1 m3 volume. The final condition is 600 kPa. Find the amount of heat added and mass of steam. Given data: p1 = 400 kPa = 4 bar x1 = 0.6 V1 = 0.1 m3 p2 = 600 kPa = 6 bar To find: Q and m Solution: From saturated water table of pressure scale, corresponding to 4 bar, vf1 = 0.001084 m3/kg, vg1 = 0.4622 m3/kg hf1 = 604.7 kJ/kg, hfg1 = 2132.9 kJ/kg Specific volume, v1 = vf1 + x1(vg1 - vf1) = 0.001084 + 0.6 (0.4622 - 0.001084) = 0.278 m3/kg h1 = hf1 + x1 hfg1 = 604.7 + 0.6 × 2132.9 = 1884.44 kJ/kg Mass of steam, pppp = 0.36 kg Ans. For rigid vessel, v1 = v2 ⸫ v2 = 0.278 m3/kg From saturated water table of pressure scale, corresponding to 6 bar, vf2 = 0.001101 m3/kg, hf2 = 670.4 kJ/kg, vg2 = 0.31546 m3/kg hfg2 = 2085.1 kJ/kg, Since vg2 > v2, the steam is in wet condition. v2 = vf2 + x2(vg2 - vf2) 0.278 = 0.001101 + x2 (0.31546 - 0.001101) x2 = 0.88 ⸫ h2 = hf2 + x2 hfg2 = 670.4 + 0.88 × 2085.1 = 2505.29 kJ/kg By first law of thermodynamics, Q = W + ΔU For rigid vessel, v1 = v2. So, work transfer W = 0 ⸫ Q = ΔU Total heat transfer, Q = m[U2 - U1] = m[(h2 - p2v2) - (h1 – p1v1)] = m[(h2 – h1) - v1 (p2 - p1)] [⸪ v1 = v2] ⸫ Total heat transfer, Q = 0.36 [(2505.29 - 1884.44) - 0.278 (600 - 400)] = 203.46 kJ Ans. 2. Solved Problems on Constant Pressure Process Problem 4.11 2 kg of steam initially at 5 bar and 0.6 dry is heated at constant pressure until the temperature becomes 350°C. Find the change in entropy and internal energy. Given data: m = 2 kg p1 = 5 bar = 500 kPa x1 = 0.6 T2 = 350°C To find: ΔS and ΔU Solution: From saturated water table of pressure scale, corresponding to 5 bar, vf1 = 0.001093 m3/kg, hf1 = 640.1 kJ/kg, Sf1 = 1.86 kJ/kg K, vg1 = 0.37466 m3/kg hfg1 = 2017.4 kJ/kg, Sfg1 = 4.959 kJ/kg K Specific volume, v1 = vf1 + x1(vg1 - vf1) = 0.001093 + 0.6 (0.37466 - 0.001093) = 0.225 m3/kg Enthalpy, h1 = hf1+ x1 hfg1 = 640.1 + 0.6 × 2107.4 = 1904.54 kJ/kg Entropy, s1 = Sf1 + x1 Sfg1 = 1.86 + 0.6 × 4.959 = 4.835 kJ/kg K From superheated specific volume, superheated enthalpy and superheated entropy tables, corresponding to 5 bar and 350°C, v2 = 0.5701 m3/kg h2 = 3168.1 kJ/kg s2 = 7.634 kJ/kg K Change in entropy, ΔS= m (s2 - s1) = 2(7.634 - 4.835) = 5.598 kJ/K Ans. Change in internal energy, ΔU = m (u2 - u1) = m [(h2 – h1) - (p2v2 – p1v1)] = m[(h2 – h1) – p1 (v2 – v1)] [ ⸪ p1 = p2] = 2[(3168.1 - 1904.54) - 500(0.5701 -0.225)] = 2182.02 kJ Ans. Problem 4.12 2.5 kg of steam is heated at constant pressure of 250 kPa and 100°C until the temperature is 250°C. Using Mollier chart, find the amount of heat added and change in entropy. Given data: m = 2.5 kg Process: Constant pressure p = 250 kPa = 2.5 bar T1 =100°C T2 = 250°C To find: Q and ΔS Solution: From Mollier chart, At p = 2.5 bar and 100°C, h1 = 2700 kJ/kg and s1 = 7.04 kJ/kg K Along p = 2.5 bar line at 250°C, h2 = 2950 kJ/kg s2 = 7.65 kJ/kgK Heat added, Q = m (h2 – h1) = 2.5 (2950 - 2700) = 625 kJ Ans. Change in entropy, ΔS = m (s2 - s1) = 2.5 (7.65 - 7.04) = 1.525 kJ/K Ans. Problem 4.13 One kg of steam at a pressure of 700 kPa and 0.6 dry is heated at constant pressure until it becomes dry saturated. Determine the change in internal energy and work done. Given data: m = 1 kg p = 700 kPa = 7 bar x1 = 0.6 Process: Constant pressure x2 = 1 To find: ΔU and W Solution: From saturated water table of pressure scale, corresponding to 7 bar, vf1 = 0.001108 m3/kg, hf1 = 697.1 kJ/kg, vg1 = 0.27288 m3/kg hfg1 = 2064.9 kJ/kg, hg1 = 2762 kJ/kg, V1 = m[vf1 + x1(vg1 - vf1)] = 1[0.001108 + 0.6 (0.27288 - 0.001108)] = 0.164 m3 H1 = m(hf1 + x1 hfg1) = 1(697.1 + 0.6 × 2064.9) = 1936.04 kJ Since the final condition of steam is dry and also the pressure remains same at 7 bar, v2 = vg1 = 0.27268 m3/kg h2 = hg1 = 2762 kJ/kg V2 = m v2 = 1 × 0.27268 = 0.273 m3 H2 = m h2 = 1 × 2762 = 2762 kJ ⸫ Change in internal energy, ΔU = m(u2 - u1) = m (h2 – h1) - (p2V2 - p1V1)] = m (h2 – h1) - p1 (V2 – V1) [⸪ p1 = p2] = 1 × (2762 - 1936.04) - 700 (0.273 - 0.164) = 749.66 kJ Ans. Work done, W = p(V2 - V1) = 700 (0.273 - 0.164) = 76.3 kJ Ans. Problem 4.14 Steam at 15 bar and 300°C is expanded hyperbolically to a pressure of 5 bar. Calculate the change in internal energy and work done during the process. Given data: p1 = 15 bar = 1500 kPa T1 = 300°C = 573 K p2 = 5 bar = 500 kPa To find: ΔU and W Solution: From superheated enthalpy and specific volume tables, corresponding to 15 bar and 300°C, h1 = 3038.9 kJ/kg v1 = 0.1697 m3/kg pv = C for hyperbolic process p1v1 = p2v2 From saturated water table of pressure scale, corresponding to 5 bar, vg2 = 0.37466 m3/kg Since v2 > vg2, the steam is in superheated condition. For hyperbolic process, T2 = T1 ⸫ From superheated enthalpy table, corresponding to 5 bar and 300°C, h2 = 3064.8 kJ/kg By first law of thermodynamics, Q = W + ΔU For hyperbolic process, ΔU = 0 Q = W From hyperbolic relation, Problem 4.15 A mass of 0.9 kg of steam initially at a pressure of 1.5 MPa and temperature of 250°C expands to 150 kPa. Assume the process as isentropic. Find the condition of steam and work transfer. Given data: m = 0.9 kg p1 = 1.5 MPa = 15 bar = 1500 kPa T1 = 250°C p2 = 150 kPa = 1.5 bar Process: Isentropic To find: Condition of steam and work transfer Solution: From superheated specific volume, enthalpy and entropy tables, corresponding to p1 = 15 bar and T1 = 250°C, v1 = 0.152 m3/kg h1 = 2923.5 kJ/kg s1 = 6.71 kJ/kg K For isentropic process, s1 = s2 s2 = 6.71 kJ/kg K From saturated water table of pressure scale, corresponding to 1.5 bar, hf2 = 467.1 kJ/kg, sf2 = 1.433 kJ/kg K, hfg2 = 2226.2 kJ/kg, sfg2 = 5.79 kJ/kg K, sg2 = 7.223 kJ/kg K, vf2 = 0.001053 m3/kg, vg2 = 1.159 m3/kg Since sg2 > s2, the steam is in wet condition. s2 = sf2 + x2 sfg2 6.71 = 1.433 + x2 × 5.79 x2 = 0.911 dry Ans. ⸫ h2 = hf2 + x2 hfg2 = 467.1 + 0.911 × 2226.2 = 2495.17 kJ/kg Also, we know that v2 = vf2 + x2(vg2 - vf2) = 0.001053 + 0.911 (1.159 - 0.001053) = 1.056 m3/kg By first law of thermodynamics, Q = W + ΔU For isentropic process, Q = 0 ⸫ Work transfer, W = - ΔU = - (U2 - U1) = U1 - U2 = m[(h1 – p1v1) - (h2 – p2v2)] = m[(h1 – p1v1) - (p1v1 - p2v2)] = 0.9[(2923.5 - 2495.17) - (1500 × 0.152 – 150 × 1.056)] = 322.86 kJ Ans. Internal energy, ΔU = -W = -322.86 kJ Ans. Problem 4.16 A steam at 10 bar and 0.85 dry expands according the law of pV1.2 = C to final pressure of 1 bar. Find the final volume and final enthalpy. Given data: p1 =10 bar = 1000 kPa x1 = 0.85 p2 = 1. bar = 100 kPa Expansion according to pV1.2 = C To find: Final volume and final enthalpy Solution: From saturated water table of pressure scale, corresponding to 10 bar, Problem 4.17 Two kg of steam at a pressure of 8 bar occupies a volume of 0.3 m3. If the air expands to a volume of 1.5 m3 according to the law pV1.35 = C. Calculate the work done, heat transferred and change in entropy during the process. Given data: m = 2 kg p1 = 8 bar = 800 kPa V1 = 0.3 m3 Expansion: pV1.35 = C V2 =1.5 m3 To find: Work done (W), Heat transferred (Q) and Change in entropy (ΔS) Solution: Since there is no pressure value of 0.91 bar in the saturated water table of pressure scale, the following interpolation calculations are carried out to find specific volume corresponding to 0.91 bar. Corresponding to 0.9 bar, vg = 1.8691 m3/kg Corresponding to 0.95 bar, vg = 1.7771 m3/kg Problem 4.18 Steam is expanded as per the law pV1.15 = C from 10 bar and 0.92 dry to 3 bar. Find the work done and heat transfer from a non-flow system. Given data: pv1.15 = C p1 = 10 bar =1000 kPa x1 = 0.92 p2 = 3 bar = 300 kPa To find: W and Q Solution: From saturated water table of pressure scale, corresponding to 10 bar, Problem 4.19 One kg of steam at a pressure of 9 bar with the temperature of 200°C throttled to a pressure of 1 bar. Find the quality of steam and entropy change during the process. Given data: m = 1 kg p1 = 9 bar = 900 kPa T1 = 200°C p2 = 1 bar = 100 kPa Throttling process To find: Quality of steam and entropy change Solution: From saturated water table of pressure scale, corresponding to 9 bar, Tsat = 175.4°C, Since T1 > Tsat, the steam is in superheated condition. From superheated enthalpy and superheated entropy tables, at 9 bar and 200°C, h1 = 2832.7 kJ/kg s1 = 6.751 kJ/kg K For throttling process, there is no change in enthalpy. ⸫ h2 = h1 = 2832.7 kJ/kg From saturated water table of pressure scale, corresponding to 1 bar, hg2 = 2675.4 kJ/kg Tsat = 99.63°C Since h2 > hg2, the steam is in superheated condition. From the superheated enthalpy table, corresponding to 1 bar and h2 = 2832.7 kJ/kg, T2 = 178.46°C (By linear interpolation) Degree of super heat, (Tsup - Tsat) = 178.46 - 99.63 = 78.83°C Corresponding to 178.46°C at p2 = 1 bar from superheated entropy table, Entropy, s2 = 7.7398 kJ/kgK (By linear interpolation) Entropy change during the process, s2 – s1 = 7.7398 - 6.751 = 0.989 kJ/kgK Ans. Problem 4.20 A sample of steam from a boiler drum at 1.5 MPa is put through a throttling calorimeter in which the pressure and temperature are found to be 100 kPa, 100°C. Find the quality of the sample taken from the boiler. Given data: p1 =1.5 MPa = 15 bar = 1500 kPa p2 = 100 kPa = 1 bar T2 = 100°C To find: Quality of sample, x1 Solution: From saturated water table, corresponding to 15 bar, hf1 = 844.6 kJ/kg, hfg1 = 1945.3 kJ/kg hg1 = 2789.9 kJ/kg From saturated water table of pressure scale, corresponding to 1 bar, Tsat = 99.6°C, Since T2 > Tsat, the steam is in superheated condition. From the superheated enthalpy table, corresponding to 1 bar and 100°C, h2 = 2676.2 kJ/kg For throttling process, h1 = h2 = 2676.2 kJ/kg It is less than hg1, So, the steam is in wet condition. For wet steam, h1 = hf1 + x1 hfg1 2676.2 = 844.6 + x1 (1945.3) x1 = 0.94 Ans. Problem 4.21 Steam at 1 MPa and 0.9 dry is throttled to a pressure of 200 kPa. Using Steam table, find the quality of steam and change in entropy. Check your answer using Mollier Chart. State whether this process is reversible or irreversible. Given data: p1 = 1 MPa = 10 bar = 1000 kPa x = 0.9 Throttling process p2 = 200 kPa = 2 bar To find: (i) Quality of steam, x2 (ii) Change in entropy, Δs Solution: During throttling process; enthalpy remains constant, h2 = h1 From saturated water table of pressure scale, corresponding to 10 bar, From Mollier chart, for x1 = 0.9 at 10 bar line note the entropy of steam, s1 = 6.1 kJ/kg K Since the throttling process is constant enthalpy process, draw horizontal line in the Mollier chart up to 2 bar pressure line as shown in Figure 4.16. Now, the entropy of final state of steam is noted. s2 = 6.76 kJ/kg K Change in entropy, s2 - s1 = 0.66 kJ/kgK Ans. Since As is positive, the process is irreversible. Ans. Problem 4.22 A sample of steam from a boiler drum at 2 MPa is passed through a throttling calorimeter after which the pressure and temperature are found to be 1 bar and 150°C. (a) Find the dryness fraction of steam taken from the boiler. (b) If the calorimeter must show at least 8°C superheat for acceptable results, what is the minimum dryness fraction that could be measured from the given pressures? Given data: p1 = 2 MPa = 20 bar = 2000 kPa p2 = 1 bar = 100 kPa T2 = 150°C Degree of superheat = 8°C To find: x1 and minimum dryness fraction for 8°C superheat, xmin Solution: Case a: From superheated enthalpy table, corresponding to 1 bar and 150°C, h2 = 2776.3 kJ/kg For throttling process, h1 = h2 = 2776.3 kJ/kg From saturated water table of pressure scale, corresponding to 20 bar, hf1 = 908.6 kJ/kg, hg1 = 2797.3 kJ/kg hfg1 = 1888.7 kJ/kg h1 < h2. So, the steam is in wet condition. For wet steam, h1 = hf1 + x1 hfg1 2776.3 = 908.6 + x1 × 1888.7 x1 = 0.989 Ans. Case b: From saturated water table of pressure scale, corresponding to 1 bar, Tsat = 99.63°C T2 = Tsat + Tsup = 99.63 + 8 = 107.63°C From superheated enthalpy table, corresponding 1 bar and 107.63°C, h2 = 2691.48 kJ/kg (By interpolation) h1 = h2 = 2691.48 kJ/kg we know that h1 = hf1 + x1 hfg1 2691.48 = 908.6 + x1 × 1888.7 x1 = 0.944 Ans. Problem 4.23 A steam initially at a pressure of 15 bar and 0.95 dry expands isentropically to 7.5 bar and is then throttled until it is dry. Determine per kg of steam: (i) Change in entropy (ii) Change in enthalpy and (iii) Change in internal energy. Given data: p1 = 15 bar = 1500 kPa x1 = 0.95 Process: Isentropic p2 = 7.5 bar = 750 kPa Then throttled until it is dry (h2 = h3) To find: (i) Δs (ii) Δh and (iii) Δu Solution: From Mollier diagram at pressure 15 bar and dryness fraction 0.95 (point 1), h1 = 2680 kJ/kg, v1 = 0.1318 m3/kg and s1 = 6.2 kJ/kg K Since the steam expands isentropically, draw a vertical line from point 1 to 7.5 bar pressure curve as shown in Figure 4.17. This is point 2. The steam properties are noted. h2 = 2560 kJ/kg, s2 = s1 = 6.2 kJ/kg K Then steam is throttled until it is dry. Therefore, draw a horizontal line from point 2 until it reaches saturated steam (dry steam) curve and mark this point as point 3. The steam properties at point 3 are noted as follows. h3 = h2 = 2560 kJ/kg, s3 = 8.33 kJ/kg K, p3 = 0.055 bar = 5.5 kPa, v3 = 32 m3/kg Change in entropy, Δs = s3 - s1 = 8.33 - 6.2 = 2.13 kJ/kg K Ans. Change in enthalpy, Δh = h1 - h3 = 2680 - 2560 = 120 kJ/kg Ans. Change in internal energy, Δu = u3 - u1 = (h3 - h1) - (p3v3 – p1v1) = (2560 - 2680) - (5.5 × 32 – 1500 × 0.1318) = -98.3 kJ/kg Ans.1. Solved Problems on Constant Volume Process

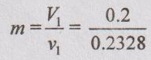 = 0.859 kg
= 0.859 kg

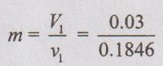 = 0.163 kg
= 0.163 kg










3. Solved Problem on Hyperbolic Process
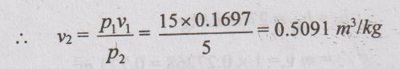
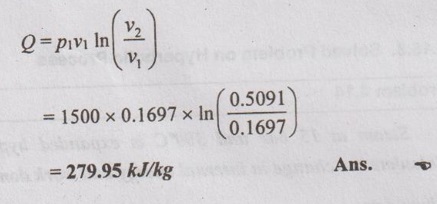
4. Solved Problem on Isentropic Process



5. Solved Problems on Polytropic Process




6. Solved Problems on Throttling Process


 Generally, throttling process is an irreversible process.
Generally, throttling process is an irreversible process.



7. Solved Problem on Combined Isentropic and Throttling Process




Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Thermodynamics - Solved Problems on Non-Flow Processes
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
