Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Solved Problems on Flow Processes
Thermodynamics
Solved Problems on Flow Processes: Properties of Pure Substances - Engineering Thermodynamics
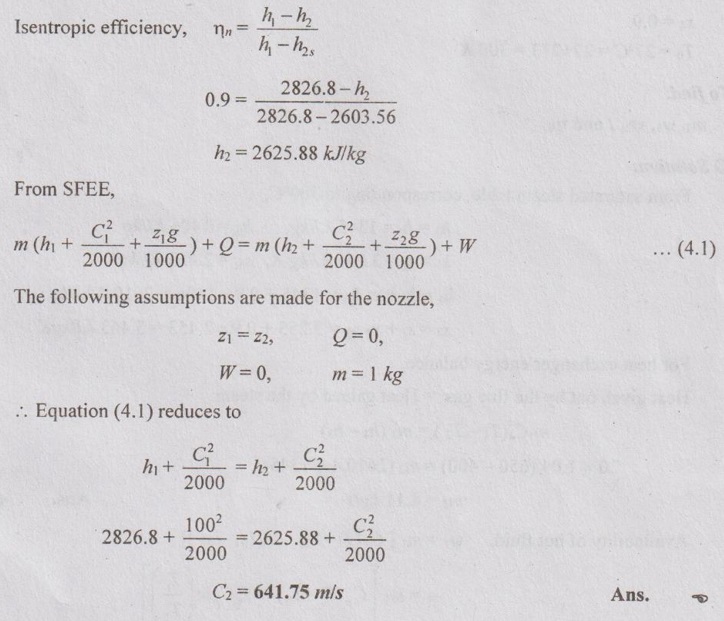
SOLVED PROBLEMS ON FLOW PROCESSES Problem 4.24 A boiler generates steam at 3 bar and 0.85 dry from water at 45°C; 540 kJ/s heat is added during the evaporation. Calculate the amount of steam generated per hour. Given data: p = 3 bar = 300 kPa x = 0.85 Tw = 45°C Heat added = 540 kJ/s To find: Steam generated per hour Solution: From saturated water table of pressure scale, corresponding to 3 bar, Problem 4.25 Steam enters an adiabatic turbine at 10 MPa and 500°C at the rate of 3 kg/s and leaves at 50 kPa. If the power output of turbine is 2 MW, determine the velocity of steam at exit of turbine. Given data: p1 = 10 MPa = 100 bar T1 = 500°C m = 3 kg/s p2 = 50 kPa = 0.5 bar W = 2 MW = 2000 kW To find: C2 Solution: From superheated enthalpy and superheated entropy tables, corresponding to 100 bar and 500°C, h1 = 3374.6 kJ/kg s1 = 6.599 kJ/kg K In adiabatic process, entropy remains constant, s1 = s2 ⸫ s2 = 6.599 kJ/kg K From saturated water table of pressure scale, corresponding to 0.5 bar, Problem 4.26 150 kg/s of steam at 25 bar and 300°C expands isentropically in a steam turbine to 0.33 bar. Determine the power output of the turbine. Given data: m = 150 kg/s p1 = 25 bar = 2500 kPa T1 = 300°C p2 = 0.33 bar = 33 kPa To find: W Solution: From superheated enthalpy and superheated entropy tables, corresponding to 25 bar and 300°C, h1 = 3010.4 kJ/kg s1 = 6.648 kJ/kgK For isentropic process, s1 = s2 ⸫ s2 = 6.648 kJ/kg K From saturated water table of pressure scale, corresponding to 0.33 bar, hf2 = 298.6 kJ/kg, sf2 = 0.971 kJ/kg K, hfg2 = 2330.6 kJ/kg, s fg2 = 6.766 kJ/kg K s2 = sf2 +x2 sfg2 From saturated water table of pressure scale, corresponding to 0.33 bar, 6.648 = 0.971 + x2 × 6.766 x2 = 0.839 h2 = hf2 + x2 hfg2 = 298.6 + 0.839 × 2330.6 = 2253.97 kJ/kg Turbine work, W = m (h1 - h2) = 150 (3010.4 - 2253.97) = 113464.5 kW = 113.47 MW Ans. Problem 4.27 A nozzle is supplied with steam of 1 MPa at 200°C with a velocity of 100 m/s. The expansion takes place to a pressure of 300 kPa. Assuming isentropic efficiency of nozzle to be 90%, find the final velocity. Given data: p1 = 1 MPa = 10 bar = 1000 kPa T1 = 200°C C1 = 100 m/s p2 = 300 kPa = 3 bar ηisentropic = 90% To find: C2 Solution: From saturated water table of pressure scale, corresponding to 10 bar, Tsat = 179.9°C Since T1 > Tsat, the state would be in the superheated region. From superheated enthalpy and superheated entropy tables, at 10 bar and 200°C, h1 = 2826.8 kJ/kg s2 = 6.692 kJ/kgK, From saturated water table of pressure scale, corresponding to 3 bar, hf2 = 561.5 kJ/kg, hfg2 = 2163.2 kJ/kg sf2 = 1.672 kJ/kgK, sfg2 = 5.319 kJ/kgK s2s = sf2 + x2 sfg2 s1= s2s = 6.692 kJ/kgK ⸫ 6.692 = 1.672 + x2s × 5.319 x2s = 0.944 ⸫ h2s = hf2 + x2s hfg2 = 561.5 + 0.944 × 2163.2 = 2603.56 kJ/kg Problem 4.28 In a steam generator, the steam generating tubes receive heat from hot gases passing over the oxide surface evaporating water inside the tubes. Flue gas flow rate is 20 kg/s with an average specific heat of 1.04 kJ/kgK. The gas temperature decreases from 650°C to 400°C while generating steam at 300°C water enters the tube as a saturated liquid and leaves with a quality of 90%. Assume environment temperature as To = 27°C. Determine the water flow rate, availability of hot fluid and cold fluid, irreversibility and second law efficiency. Given data: m1 = 20 kg/s C1 = 1.04 kJ/kg K T1 = 650°C = 650 + 273 = 923 K T2 = 400°C 400 + 273 = 673 K T3 = 300°C (Saturated liquid) x4 = 0.9 To = 27°C = 27 + 273 = 300 K To find: m2, ψ1, ψ2, I and ηII Solution: From saturated steam table, corresponding to 300°C, Problem 4.29 Steam flows in a pipeline at 1.5 MPa. After expanding to 0.1 MPa in a throttling calorimeter, the temperature is found to be 120°C. Find the quality of steam in the pipeline and also calculate availabilities, irreversibility and second law efficiency. Assume To = 25°C. Given data: p1 =1.5 MPa = 15 bar p2 = 0.1 MPa = 1 bar T2 = 120°C = 120 + 273 = 393 K To = 25°C = 25 + 273 = 298 K To find: x1, ψ1, ψ2, I and ηII Solution: From the superheated steam table, corresponding to p2 = 1 bar and T2 = 120°C, h2 = 2716.2 kJ/kg s2 = 7.4606 kJ/kgK For throttling process, h1 = h2 = 2716.2 kJ/kg But at p1 = 15 bar, h1 = 844.89 kJ/kg; sf = 2.3315 kJ/kgK; hfg = 1947.3 kJ/kg sfg = 6.4448 kJ/kgK h1 = hf + x1 hfg 2716.2 = 844.89 + x1 × 1947.31. Solved Problem on Boiler

2. Solved Problems on Turbine


3. Solved Problems on Nozzle


Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Thermodynamics - Solved Problems on Flow Processes
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
