Engineering Thermodynamics: Unit IV: Properties of Pure Substances
p-v-T Surface
Pure Substances | Thermodynamics
The three important thermodynamic properties such as pressure (p), specific volume (v) and temperature (T) are plotted in three dimensional coordinates.
p-v-T SURFACE The three important thermodynamic properties such as pressure (p), specific volume (v) and temperature (T) are plotted in three dimensional coordinates. This plot is called p-v-T surface. Here, T and v may be viewed as the independent variables (horizontal surface) and p as the dependent variable (vertical surface). Figure 4.8 (a) represents the p-v-T surface for the substances which expand upon freezing similar to water and Figure 4.8 (b) represents the p-v-T surface for the substances which contract upon freezing. All two dimensional diagrams (except h-s diagram) are merely projections of this three dimensional plot onto the appropriate planes. For example, p-v diagram is a projection of the p-v-T surface on p-v plane. Eventhough the p-v-T surface provides a great deal of information, it is more convenient to work with two-dimensional diagrams such as p-v, p-T and T-v diagrams.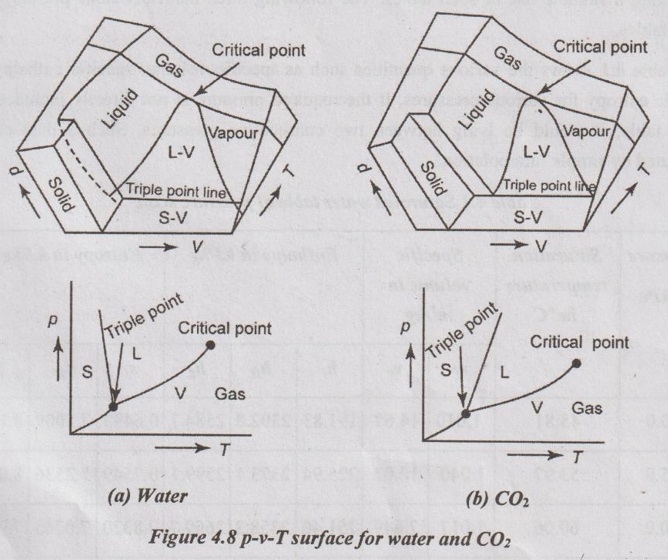 All points on the surface represent equilibrium states. The single phase regions appear as curved surfaces on the p-v-T surface and the two phase regions as surfaces perpendicular to p-T plane.
All points on the surface represent equilibrium states. The single phase regions appear as curved surfaces on the p-v-T surface and the two phase regions as surfaces perpendicular to p-T plane.
Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Pure Substances | Thermodynamics - p-v-T Surface
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
