Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Formation of steam and its Thermodynamic Properties
Consider one kg of water in a closed vessel under a pressure of p (N/m2) and at a temperature of – 20°C.
FORMATION OF STEAM AND ITS THERMODYNAMIC PROPERTIES Consider one kg of water in a closed vessel under a pressure of p (N/m2) and at a temperature of – 20°C. (a) The temperature of the ice will increase till it reaches the freezing temperature of water i.e. 0°C. It is shown by the line 1-2 in Figure 4.1. (b) When more heat is added after the point 2, the ice starts melting at the same time. There is no rise in temperature till the whole of the ice has been melted and converted into water. This process is represented by a line 2-3 shown in Figure 4.1. The heat added during this period is called latent heat of fusion of ice or latent heat of ice. (c) On further heating, the water reaches its boiling point or saturation point 4. At a given pressure, the temperature at which a pure substance starts boiling is called saturation temperature, Tsat. Similarly, at a given temperature, the pressure at which a pure substance starts boiling is called saturation pressure, psat. Both saturation temperature and pressure are the function of each other. At atmospheric pressure, the boiling point of water is 100°C. The amount of heat added during heating of water from 0°C to saturation temperature of 100°C is known as sensible heat. It is denoted by hf. Mathematically, h = m Cp (T2 - T1) (d) On further heating beyond 4, the water will gradually be converted into steam when the temperature remains constant. Steam is the substance in which the evaporation is not completed in from its liquid state. At this stage, the steam will have some water particles in suspension called wet steam. The same process continues till all water particles converted into wet steam. The line 4-5 in Figure 4.1 represents this process. The amount of heat added during heating of water from boiling point to dry saturated stage is called latent heat of vaporization or enthalpy of vaporization or latent heat of steam. It is denoted by 'hfg'. (e) If the water is further heated, the water particles in suspension will be fully converted into steam. The whole steam without any water suspension is called dry steam or dry saturated steam. (f) When the dry steam is further heated, the temperature rises again. This process is called superheating and the steam obtained is known as superheated steam. The heat supplied to the dry steam at saturation temperature Ts (i.e. 100°C), to convert it into superheated steam at the temperature Tsup is called heat of super heat or superheat enthalpy. It is denoted by 'hsup'. Dryness fraction is defined as the ratio of the mass of the dry steam actually present to the mass of the total steam. It is denoted by 'x'. The dryness fraction when it is expressed in percentage (i.e. 100 x) is called quality of steam. It is defined as the ratio of the mass of water vapour in suspension to the total steam. The wetness fraction expressed in percentage i.e. 100(1 - x) is called priming. The number of independent variables associated with a multi-component, multiphase system is given by the phase rule. It is also called Gibbs phase rule. It is expressed by the equation n = C – ϕ + 2 where n = Number of independent variable C = Number of components ϕ = Number of phases present in equilibrium. For the single component (C = 1) two phase system (ϕ = 2) system, the number of independent variable needed is calculated by n = 1 – 2 + 2 = 1 For example, the water (C = 1) at the triple point has 3 phases (ϕ = 3) and thus, n = 0. It means, none of the properties of a pure substance at the triple point can be varied. From this rule, it can be stated that a pure substance which exists in a single phase (ϕ = 1) will have two independent variables (n = 2). It means, there are two independent intensive properties required to be specified to fix up the state of the system at equilibrium. If the water is gradually heated when the pressure remains constant, the following changes will occur:
If the water is gradually heated when the pressure remains constant, the following changes will occur:
1. Dryness Fraction
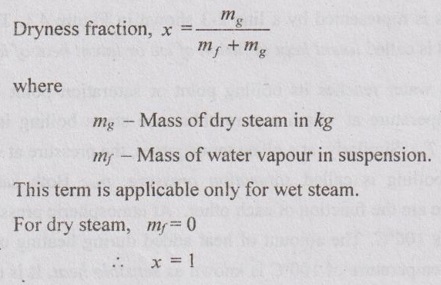
2. Wetness Fraction
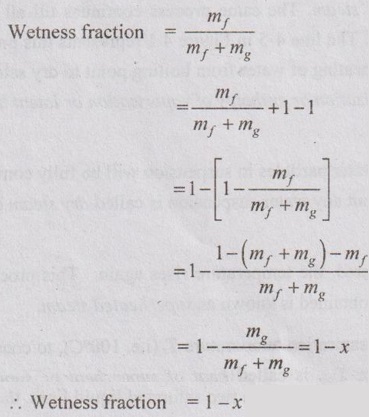
3. Phase Rule
Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : - Formation of steam and its Thermodynamic Properties
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
