Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Entropy Relations (Tds Equations)
Thermodynamics
The entropy (s) of a pure substance can be expressed as a function of temperature (T) and pressure (p).
ENTROPY RELATIONS (Tds EQUATIONS) Entropy as a function of T and p: The entropy (s) of a pure substance can be expressed as a function of temperature (T) and pressure (p). We know that from equation (5.69), It is known as the first form of entropy equation (or) the first Tds equation. By considering the entropy of a pure substance as a function of temperature (T) and specific volume (v), From Maxwell relation, Substituting the above expressions in ds equation, It is known as the second form of entropy equation (or) the second Tds equation. As per Theorem 3 of exact differentials (Page 5.31), the entropy (s) of a pure substance can be expressed as a function of pressure (p) and specific volume (v). i.e. s = s (p, v) By mathematical rule, Substituting (5.75) and (5.76) in equation (5.74), Tds = Cp dT + Cv dT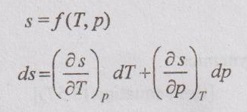
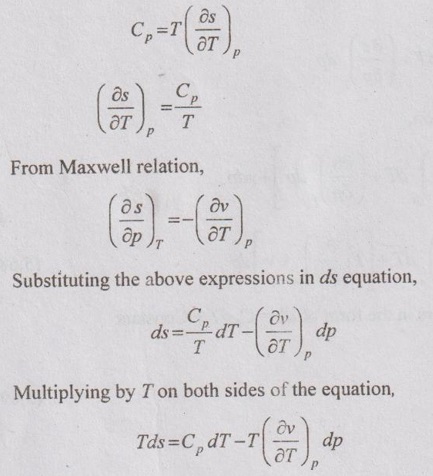
Entropy as a function of T and v:
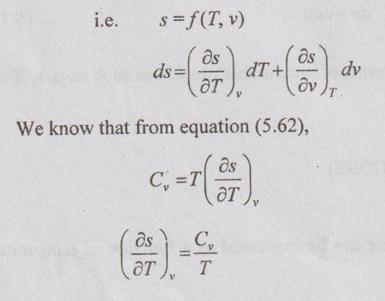
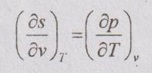

Entropy as a function of p and v:
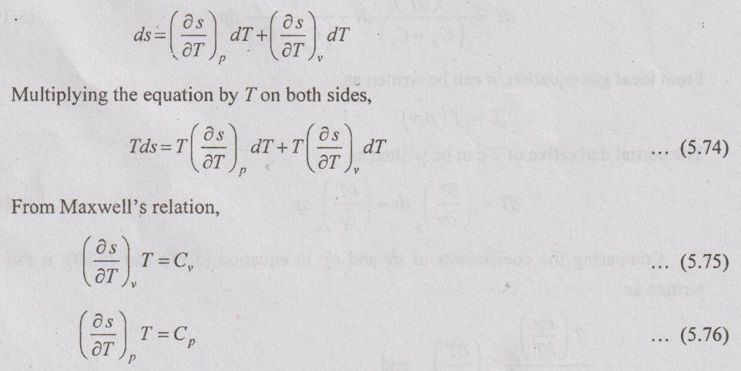
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Thermodynamics - Entropy Relations (Tds Equations)
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
