Engineering Thermodynamics: Unit II: Second Law and Entropy
Entropy: a Property of the System
Let, a thermodynamic system undergoes a change of state from 1 to 2 by a reversible process 1-A-2 and returns to its original state 1 by another reversible process 2-B-1 and completing a cycle 1-2-1.
ENTROPY: A PROPERTY OF THE SYSTEM Let, a thermodynamic system undergoes a change of state from 1 to 2 by a reversible process 1-A-2 and returns to its original state 1 by another reversible process 2-B-1 and completing a cycle 1-2-1. For this cyclic reversible process, the entropy equation is given by Now, let us consider the cycle 1-2-1 completed by another reversible process 2-C-1. i.e., it is independent of the path and a function of end states only. Hence, the entropy is a property of a system.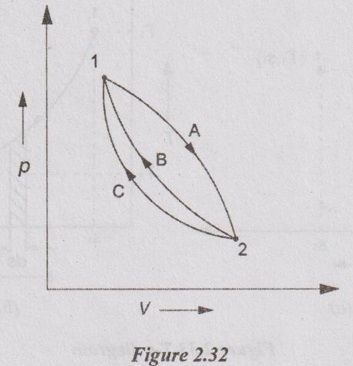

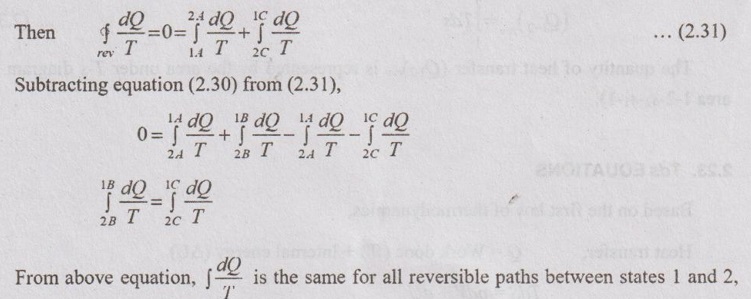
Engineering Thermodynamics: Unit II: Second Law and Entropy : Tag: : - Entropy: a Property of the System
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
