Engineering Physics: Unit III: c. Lasers
Carbon Dioxide Laser
Working Principle, Construction, Characteristics, Advantages, Disadvantages, Applications
The first molecular CO2, gas laser was developed by Indian born American Scientist Prof. C.K.N. Patel.
Molecular Gas Laser
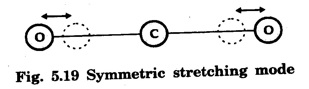
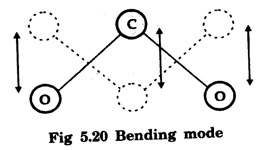
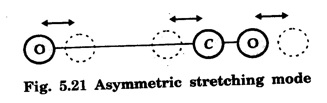
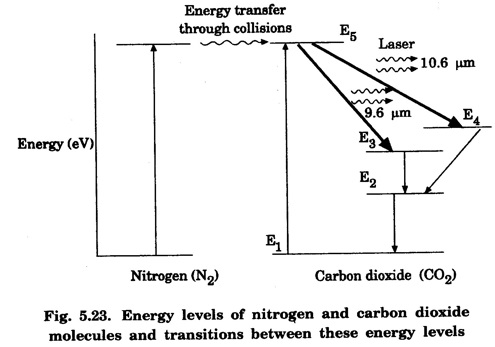
In a molecular gas laser, laser action takes place by transitions between vibrational and rotational energy levels of gas molecules. CARBON DIOXIDE (CO2) LASER The first molecular CO2, gas laser was developed by Indian born American Scientist Prof. C.K.N. Patel. It is a four-level molecular gas laser. In this laser, transition takes place between vibrational energy states of carbon dioxide molecules. It is a very efficient laser. A carbon dioxide molecule has a carbon atom at the centre with two oxygen atoms attached, one at each sides. Such a molecule vibrates in three independent modes. They are (a) Symmetric stretching mode (b) Bending mode (c) Asymmetric stretching mode In this mode of vibration, carbon atom is at rest. Both oxygen atoms vibrate such that they are moving away or approaching the fixed carbon simultaneously along the axis of the molecule (Fig. 5.19). In this mode of vibration, both oxygen atoms and carbon atom vibrate perpendicular to molecular axis (Fig. 5.20). In this mode of vibration, both oxygen atoms and carbon atom vibrate asymmetrically, i.e., oxygen atoms move in one direction while carbon atom moves in the opposite direction (Fig. 5.21). The laser transition takes place between the vibrational energy states of CO2, molecules. It consists of a quartz discharge tube 5 m long and 2.5 cm in diameter (Fig. 5.22). This discharge tube is filled with the gas mixture of CO2, nitrogen and helium with suitable partial pressures. The terminals of the discharge tube are connected to D.C. power supply. The ends of this tube are fitted with NaCl Brewster windows so that the laser light generated is plane polarised. The optical resonator is formed with two concave mirrors one fully reflecting M1 with the other partially reflecting M2. The energy level diagram of nitrogen and carbon dioxide molecules is shown in fig 5.23. When the electrical discharge occurs in gas mixture, the electrons collide with nitrogen molecules and they are raised to excited energy states. This process is represented by the equation N2 → Nitrogen molecule in ground state e* →Electron with high energy N2 * → Nitrogen molecule in excited state e → Same electron with lesser energy Since excited energy level of nitrogen is very close to E5 energy level of CO2 molecules, CO2 molecules are excited by energy transfer and population inversion is achieved. This process is represented by the equation N2 * → Nitrogen molecule in excited state CO2 → Carbon dioxide molecule in ground state CO2 * → Carbon dioxide molecule in excited state N2 → Nitrogen molecule in ground state Any of the spontaneously emitted photon triggers laser action in the tube. There are two possible types of laser transition. (i) Transition E5-E4 This transition produces a laser beam of wavelength 10.6 μm. (ii) Transition E5 – E3 This transition produces a laser beam of wavelength 9.6 μm. Normally 10.6 μm transition is more intense than 9.6 um transition. The power output from this laser is 10 kW. Note: The helium gas is used to conduct heat generated in the central region of the discharge tube to the walls of the discharge tube. • Type : Molecular gas and four level laser. • Active medium : A gas mixture of CO2, N2 and helium. • Pumping method : Electrical discharge method, • Optical resonator: It is formed with two concave mirrors. • Power output: 10 kW. • Nature of output: Continuous wave or pulsed wave. • Wavelength of output: 9.6 μm and 10.6 μm (96000 Å and 106000 Å infra red). • The construction of CO2 laser is simple. • The output from this laser is continuous. • It has high efficiency. • It has very high output power. • The output power can be increased by increasing the length, of discharge tube. • The contamination of oxygen by carbon monoxide has some effect on laser action. • The operating temperature plays an important role in determining the output power of the laser. • Corrosion may occur at the surfaces of the discharger tube. • Due to high power laser light, accidental exposure may damage eyes, since it is invisible (infra red region) to our eyes. • High-power CO2 laser finds application in material processing, welding, drilling, cutting, soldering, etc. • The low atmospheric attenuation (10.6 μm) makes CO2, laser suitable for open air communication. • It is used in remote sensing. • It is used in the treatment of liver and lung diseases. • It is mostly used in neurosurgery and general surgery. • It is used to perform microsurgery and bloodless operations.Energy states of CO2 molecules
(a) Symmetric stretching
(b) Bending
(c) Asymmetric stretching
Principle
Construction
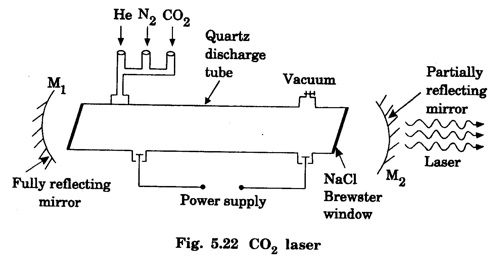
Working
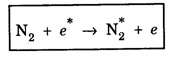
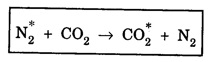
Characteristics
Advantages
Disadvantages
Applications
Engineering Physics: Unit III: c. Lasers : Tag: : Working Principle, Construction, Characteristics, Advantages, Disadvantages, Applications - Carbon Dioxide Laser
Related Topics
Related Subjects
Engineering Physics
PH3151 1st semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
