Engineering Thermodynamics: Unit IV: Properties of Pure Substances
Calculation of work done and Heat Transfer in non-flow processes
Thermodynamics
In Unit 1, the various non-flow processes are discussed in detail as applicable to gases.
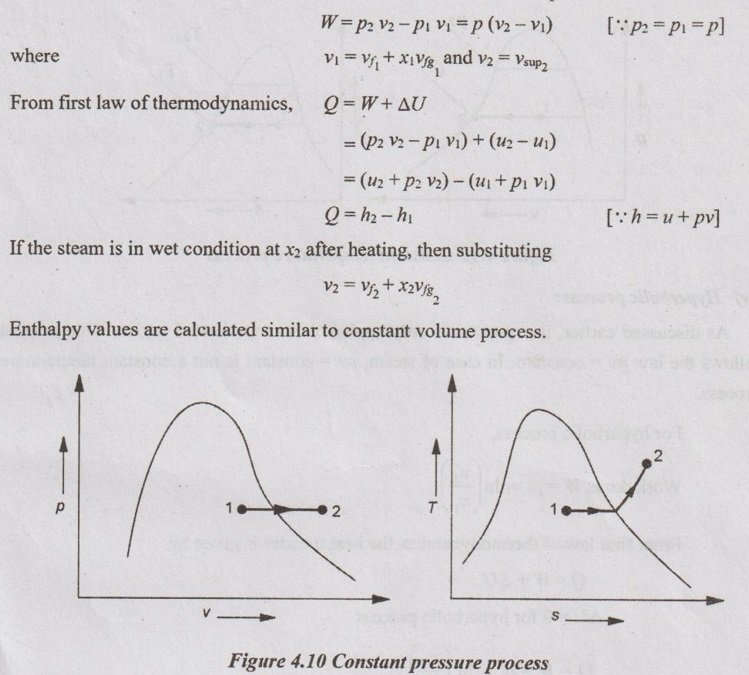
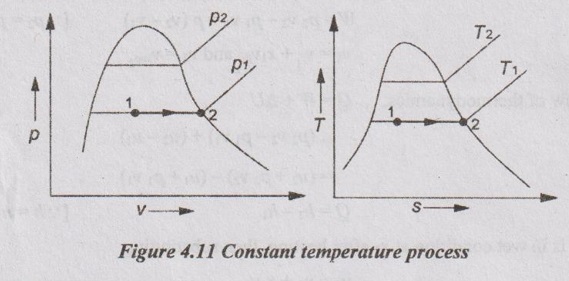
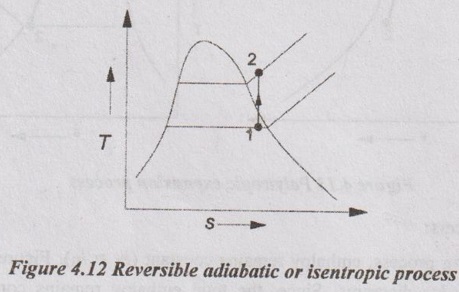
CALCULATION OF WORK DONE AND HEAT TRANSFER IN NON-FLOW PROCESSES In Unit 1, the various non-flow processes are discussed in detail as applicable to gases. In this chapter, the calculations of work done, heat transfer, enthalpy, entropy and internal energy for vapour are discussed. The foresaid properties for vapour can be calculated with the use of basic first and second law of thermodynamics equations with suitable modifications. The basic equation for non-flow process is given by Q = W + ΔU or dQ = pdv + dU The various non-flow processes are discussed below. The wet steam at state 1 with x1, p1, v1, T1 is heated to superheated steam at state 2 with p2, vsup2, Tsup2 at constant volume. Figure 4.9 shows p-v and T-s diagrams of this process. The wet steam at state 1 with x1, p1, v1, T1 is heated to superheated steam at state 2 with p2, vsup2, Tsup2 at constant pressure. Figure 4.10 shows p-v and T-s diagrams of this process. Production of steam in boiler is an example for constant pressure heating process. For constant pressure process, work done is calculated by the equation The wet steam at state 1 with x1, p1, v1, T1 is heated till it becomes dry saturated at state 2 with p2, v2, vg2 at constant temperature. Figure 4.11 shows p-v and T-s diagrams of this process. In wet region, the constant temperature process is same as constant pressure process. In the superheated region, steam behaves as gas and the constant pressure process becomes hyperbolic process. Therefore, the constant temperature process is limited to wet region only. Heat transfer, Q = h2 – h1 Work done, W = h2 – h1 [⸪ ΔU = 0] Enthalpy, h1 is calculated similar to constant volume process. But we know that h2 = hg2 (iv) Hyperbolic process: As discussed earlier, this process is only applicable to superheated region. This process follows the law pv = constant. In case of steam, pv = constant is not a constant temperature process. For hyperbolic process, During this process, the entropy remains constant (s2 = s1). It is represented by a vertical line in T-s diagram as shown in Figure 4.12. For adiabatic process, Heat transfer, Q = 0 From first law of thermodynamics, Q = W + ΔU As Q = 0 for this process, Work done, W = -ΔU = u1 - u2 = (h1 - p1 v1) - (h2 - p2 v2) = (h1 - h2) - (p1 v1 - p2 v2) In this case, v1 = vf1 + x1 vfg1 and v2 = vsup2 Enthalpy values are calculated similar to constant volume process. Polytropic process follows the law of pvn = constant. Figure 4.13 shows p-v and T-s diagrams of polytropic expansion process. Enthalpy and specific volume values are calculated similar to constant volume process. Irreversible adiabatic process is also follows the polytropic condition with an index n. In case of steam, the value of index n varies from 1.13 (for wet steam) and 1.3 (for superheated steam). During throttling process, enthalpy remains constant (h1 = h2). Figure 4.14 shows this process in T-s and h-s diagrams. Since, the total enthalpy remains constant during the process. hf1 + x1 hfg1 = hg2 + Cps (TSup2 - Ts2)(i) Constant volume process:

(ii) Constant pressure process:
(iii) Constant temperature process:
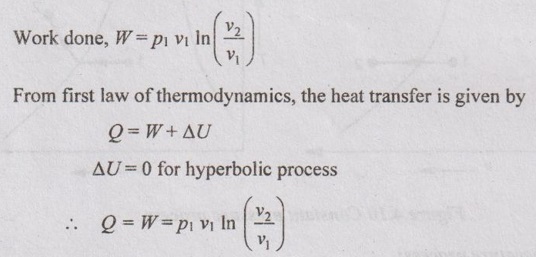
(v) Reversible adiabatic or isentropic process:
(vi) Polytropic process:

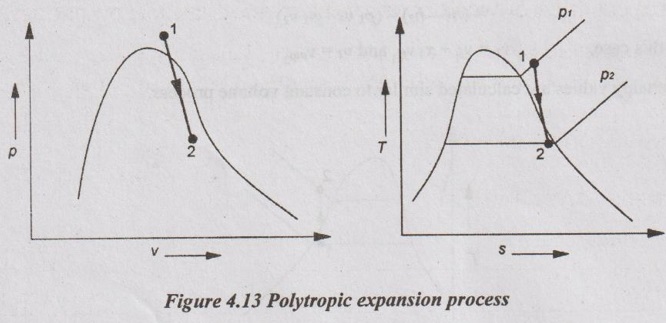
(vii) Throttling process:
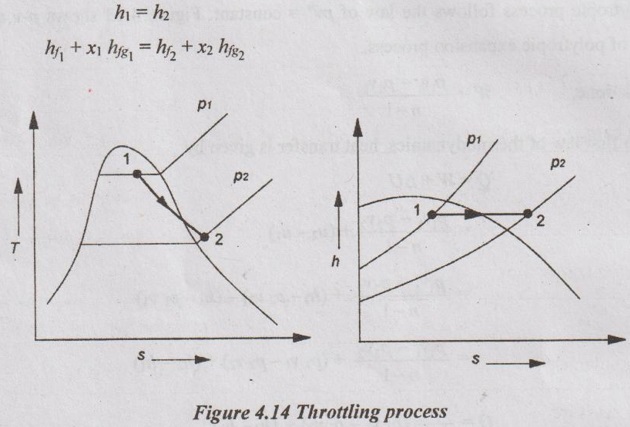 The above equation is only applicable if the state of steam before and after the throttling process will be in wet condition. If the steam is in superheated condition, then the above equation becomes
The above equation is only applicable if the state of steam before and after the throttling process will be in wet condition. If the steam is in superheated condition, then the above equation becomes
Engineering Thermodynamics: Unit IV: Properties of Pure Substances : Tag: : Thermodynamics - Calculation of work done and Heat Transfer in non-flow processes
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
