Engineering Physics: Unit IV: Basic Quantum Mechanics
Two Marks Questions with Answers
Basic Quantum Mechanics | Engineering Physics
ANNA UNIVERSITY "2' MARKS Q&A : Engineering Physics: Basic Quantum Mechanics
ANNA UNIVERSITY PART – A '2' MARKS Q&A 1. State compton effect. When a beam of X- rays is scattered by a substance of low atomic number, the scattered radiation consists of two components. One has the same wavelength λ as the incident ray and the other has a slightly longer wavelength λʹ. This phenomenon of change in wavelength of scattered X -rays is known as compton effect. 2. What is Compton wavelength? The change in wavelength corresponding to scattering angle of 90° obtained in Compton effect is called Compton wavelength. This is known as Compton wavelength of electron. 3. What are matter waves? The waves associated with moving particles of matter (e.g., electrons, photons, etc) are known as matter waves or de-Broglie waves. 4. How De-Broglie justified his concept? • Our universe is fully composed of light and matter. • Nature loves symmetry. If radiation like light can act like wave and particle, then material particles (e.g., electron, neutron etc.) should also act as particle and wave. • Every moving particle has always associated with a wave. 5. Write an expression for the wavelength of matter waves? (or) What is de - Broglie's wave equation? Wavelength for matter waves is where h → planck's constant m → mass of the particle v → velocity of the particle with which the wave is associated. p → momentum of the particle. 6. Write an expression for the de - Broglie wavelength associated with electrons. De-Broglie wave length associated with electrons accelerated by the potential V. where h → planck's constant e → charge of the electron m → mass of the electron V→ accelerating voltage 7. State the properties of the matter waves. (i) Lighter is the particle, greater is the wavelength ... associated with it. (ii) Smaller is the velocity of the particle, greater is wavelength associated with it. (iii) These waves are not electromagnetic waves. (iv) The velocity of de Broglie wave is equal to the velocity of the material particle. 8. Write down Schroedinger time independent and dependent wave equations. Schroedinger time independent wave equation is Schroedinger time dependent wave equation Ψ - Wave function m - Mass of the particle. E - Total energy of the particle. V - Potential energy. 9. Mention some of the physical significances of the wave function. (i) The wave function (Ψ) relates the particle and wave nature of matter statistically. (ii) It is a complex quantity and hence we cannot measure it. (iii) If the particle is certainly to be found somewhere in a space of dimensions dx, dy, dz, then the probability value is equal to one. 10. What are eigen values and eigen function? Energy of a particle moving in one dimensional box of width a is given by For each value of n, there is an energy level. Each value of E, is called an eigen value. For every quantum state (i.e., for different 'n' values), there is a corresponding wave function Ψn. This corresponding wave function is called eigen function. Eigen function associated with dimensional box is given by ADDITIONAL PART - A '2' MARKS Q & A 1. What is Schrodinger wave equation? The equation that describes the wave nature of a particle in mathematical form is known as Schrodinger wave equation. 2. What is a wave function? A variable quantity which characterises de - Broglie wave is known as wave function and it is denoted by the symbol Ψ. 3. Define correspondance principle. Any new theory in Physics must reduce to well-established corresponding classical theory when the new theory is applied to the special situation in which the less general theory is known to be valid.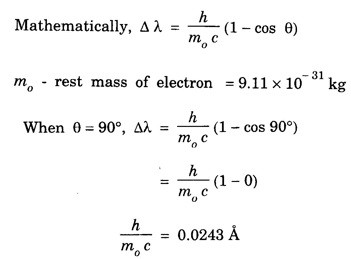
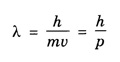
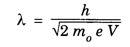
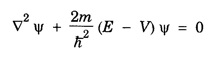
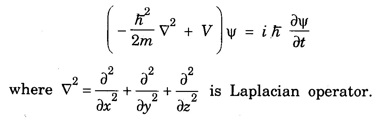
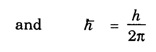
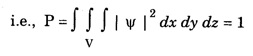
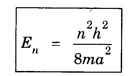
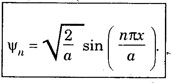
Engineering Physics: Unit IV: Basic Quantum Mechanics : Tag: : Basic Quantum Mechanics | Engineering Physics - Two Marks Questions with Answers
Related Topics
Related Subjects
Engineering Physics
PH3151 1st semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
