Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Solved Problems on Thermodynamic Relations
Solved Problems on Thermodynamic Relations: Gas Mixtures and Thermodynamic Relations - Engineering Thermodynamics
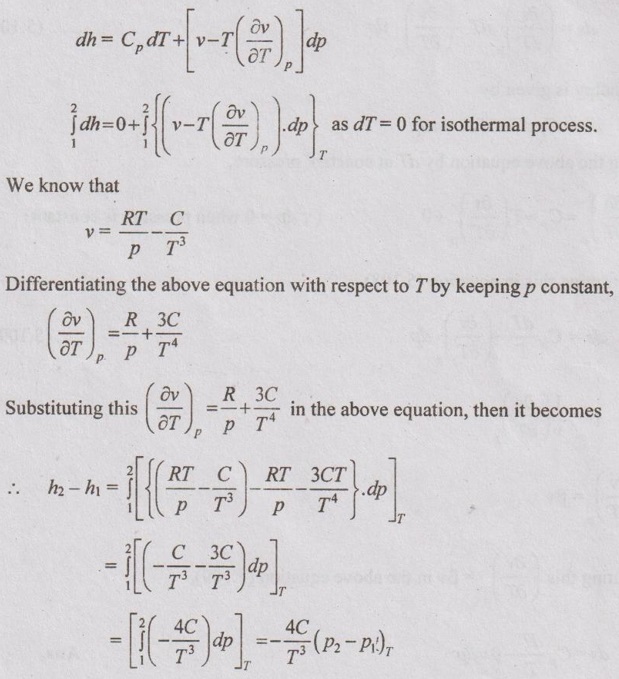
SOLVED PROBLEMS ON THERMODYNAMIC RELATIONS Problem 5.5 Obtain the expression for ds in terms of dT and dp. Solution: Let us assume, the entropy is a function of temperature and pressure. The enthalpy is given by dh = Cp dT = Tds + vdp Dividing the above equation by dT at constant pressure, ⸫ Substituting this in equation (5.108), Problem 5.6 The equation of state in the given range of pressure and temperature is given by Given data: Solution: The general equation for finding change in enthalpy (dh) is given by The general equation for finding ds is given by the equation (5.109) which is already discussed in previous example. Problem 5.7 Using Calusius Clapeyron equation, estimate the enthalpy of vaporization at 220° C saturation temperature. Take the following data: vg = 0.08603 m3/kg, vf = 0.00119 m3/kg and Given data: Ts = 220°C = 220 + 273 = 493 K vg = 0.08603 m3/kg v = 0.00119 m3/kg (dp/dT) = 35 kPa/K Solution: Using the Calusius Clapeyron equation,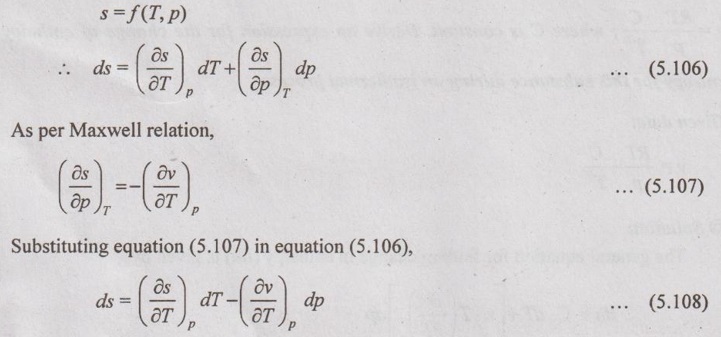
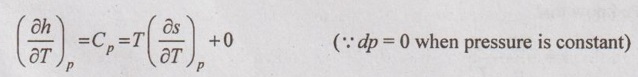
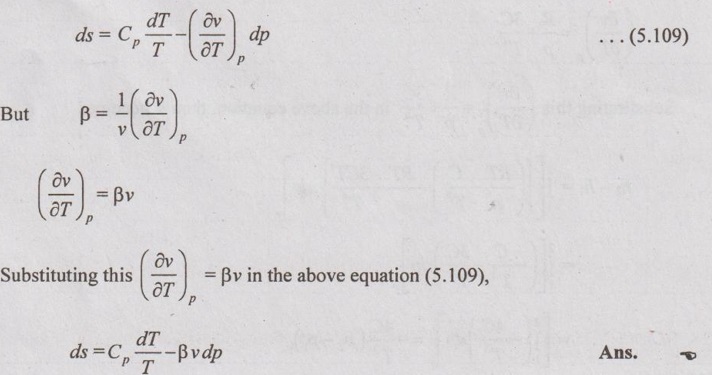
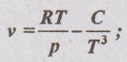 where C is constant. Derive an expression for the change of enthalpy and entropy for this substance during an isothermal process.
where C is constant. Derive an expression for the change of enthalpy and entropy for this substance during an isothermal process.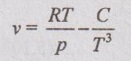

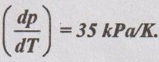
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : - Solved Problems on Thermodynamic Relations
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
