Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Solved Problems on Ideal and Real Gases
Thermodynamics
Solved Problems on Ideal and Real Gases: Gas Mixtures and Thermodynamic Relations - Engineering Thermodynamics
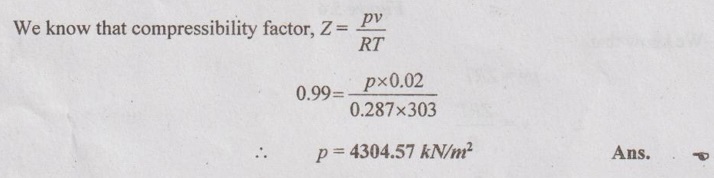
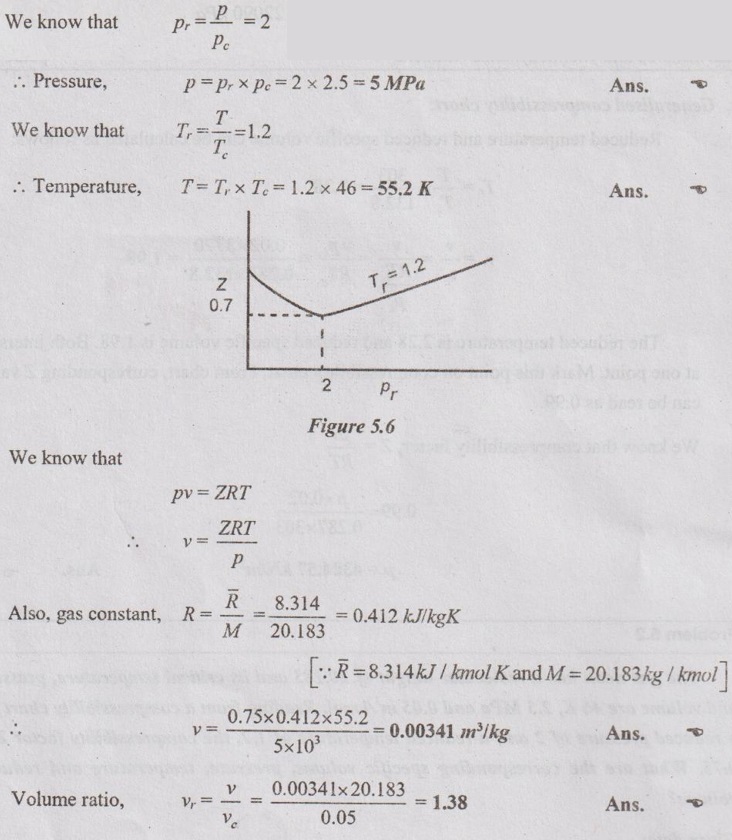
SOLVED PROBLEMS ON IDEAL AND REAL GASES Problem 5.1 A vessel of volume 0.3 m3 contains 15 kg of air at 303 K. Determine the pressure exerted by the air using 1. Perfect gas equation 2. Van der Waals equation 3. Generalised compressibility chart. Take critical temperature of air is 132.8 K and critical pressure of air is 37.7 bar. Given data: Volume, V = 0.3 m3 Mass, m = 15 kg Temperature, T = 303 K Critical temperature, (Tc) = 132.8 K Critical pressure, (pc) = 37.7 bar = 37.7 × 100 = 3770 kN/m2 Solution: pV = mRT Reduced temperature and reduced specific volume can be calculated as follows: The reduced temperature is 2.28 and reduced specific volume is 1.98. Both intersect at one point. Mark this point on compressibility chart. From chart, corresponding Z value can be read as 0.99. Problem 5.2 The gas neon has a molecular weight of 20.183 and its critical temperature, pressure and volume are 46 K, 2.5 MPa and 0.05 m3/kmol. Reading from a compressibility chart for a reduced pressure of 2 and a reduced temperature of 1.2, the compressibility factor Z is 0.75. What are the corresponding specific volume, pressure, temperature and reduced volume? Given data: Molecular weight of neon = 20.183 Critical temperature, Tc = 46 K Critical pressure, pc = 2.5 MPa Critical volume, vc = 0.05 m3/kmol Tr = 1.2 Pr = 2 Z = 0.75 Solution: Problem 5.3 Compute the specific volume of steam at 0.9 bar and 570 K using Van der Waals equation. Take critical temperature of steam as 647.3 K and critical pressure as 220.9 bar. Given data: Pressure, p = 0.9 bar = 0.9 × 100 kN/m2 = 90 kPa [⸪ 1 bar = 100 kN/m2 = 100 kPa] Temperature, T = 570 K Critical temperature, Tc = 647.3 K Critical pressure, pc = 220.9 bar = 220.9 × 100 = 22090 kPa Solution: We know that Van der Waals equation Problem 5.4 A perfect gas of 0.2 kg has a pressure of 300 kPa, a temperature of 40° C and a volume of 0.06 m3. The gas undergoes an irreversible adiabatic process to a final pressure of 400kPa and final volume of 0.15 m3, work done on the gas is 50 kJ. Find Cp and Cv. Given data: m = 0.2 kg p1 = 300 kPa T1 = 40°C = 40 + 273 = 313 K v1 = 0.06 m3 p2 = 400 kPa v2 = 0.15 m3 W = -50 kJ [Work done on the gas is negative value] Solution: We know that the perfect gas equation is written as1. Perfect gas equation:

2. Van der Waals equation:
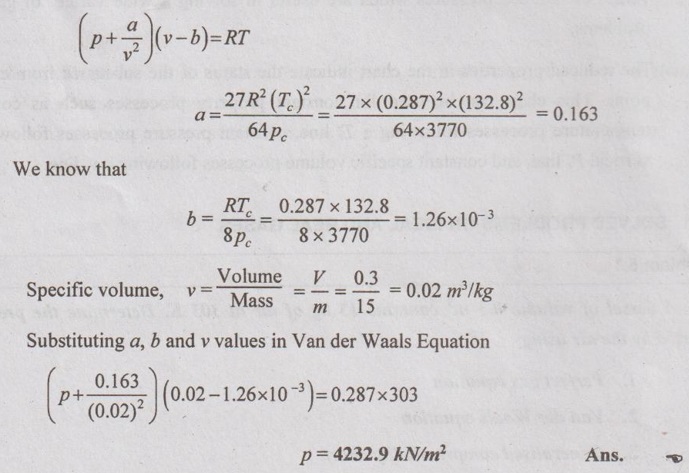
3. Generalised compressibility chart:
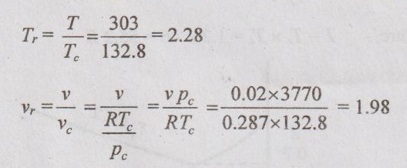
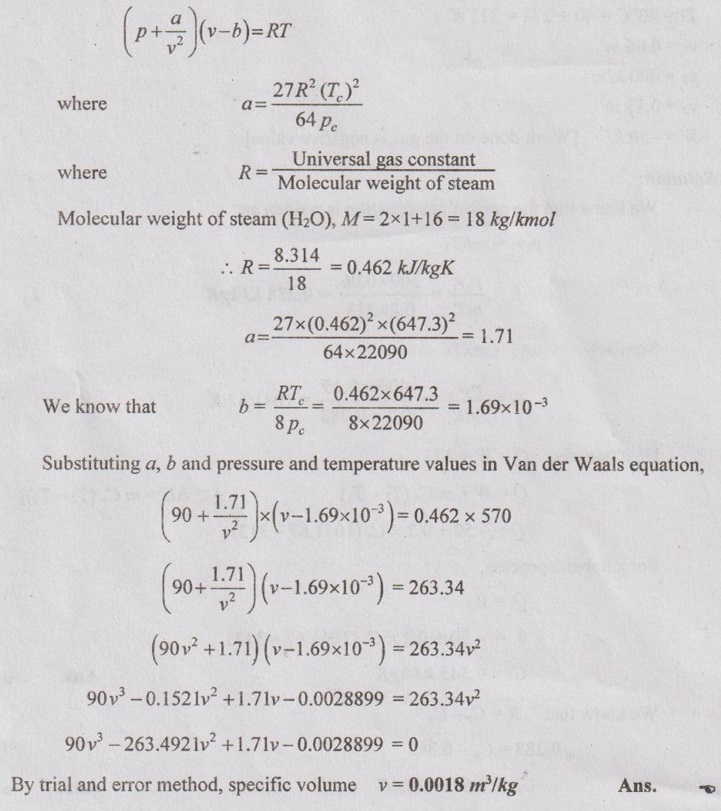

Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Thermodynamics - Solved Problems on Ideal and Real Gases
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
