Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Solved Important 16 marks Long Questions
Gas Mixtures and Thermodynamic Relations | Engineering Thermodynamics
Solved Questions: Gas Mixtures and Thermodynamic Relations - Engineering Thermodynamics
SOLVED QUESTIONS 1. State anyone equation of state for real gas and show how the deviation from ideal gas behaviour is accounted for. Refer chapter 5.2 on Page 5.2. 2. Prove that Cp of ideal gas is a function of temperature only. Refer chapter 5.2.1 on Page 5.2. 3. State the main reasons for the deviation of behaviour of real gases from ideal gases. Refer chapter 5.5 on Page 5.14. 4. Derive Van der Waals equation in terms of reduced parameters. or Deduce Van der Waals equation of state and explain its importance. Refer chapter 5.6 on Page 5.17. 5. Write the Berthelot and Dieterici equations of state. Refer Page 5.19. 6. Explain the principle of corresponding states and the use of compressibility chart. Refer chapter 5.9 on Page 5.20 for principle of corresponding states and chapter 5.10.1 on Page 5.23 for use of compressibility chart. 7. Draw a neat schematic of a compressibility chart and indicate its salient features. Refer chapter 5.10 on Page 5.21. 8. Explain reduced properties and their uses in generalized compressibility chart. List the advantages of generalized compressibility chart. Refer chapter 5.9 on Page 5.20 for reduced properties and refer chapter 5.10 on Page 5.21 for the use of reduced properties in generalized compressibility chart. 9. Derive the Maxwell relations and explain their importance in thermodynamics. Refer chapter 5.13 on Page 5.32. 10. Show that the internal energy of an ideal gas and an incompressible substance is a function of temperature only, u = u(T). Refer chapter 5.17.1 on Page 5.36. 11. Derive Maxwell's equations and write down the first and second Tds equations. Refer chapter 5.18 on Page 5.37. 12. Derive Tds equation when (i) T and V independent (ii) T and p independent (iii) p and V independent. Refer chapter 5.18 on Page 5.37. 13. Derive the Tds relation in terms of T and V and hence deduce the expression for change in the internal energy per unit change in volume at constant temperature. Refer chapter 5.18 on Page 5.37 for Tds relation in terms of T and V and refer chapter 5.17.1 on Page 5.36 for the expression for change in the internal energy per unit change in volume. 14. Prove that the difference in specific heat capacities equal to Cp – Cv = R and Refer chapter 5.19 on Page 5.40. 15. From the basic principles, prove the following Refer chapter 5.19 on Page 5.40. 16. Show that Cp – Cv = R for an ideal gas. Refer chapter 5.19 on Page 5.40. 17. Explain Joule-Thomson experiment and deduce the expression for Joule-Thomson coefficient. Or Show that the Joule-Thomson coefficient of an ideal gas is zero. Refer chapter 5.21 on Page 5.44. 18. Explain Joule-Kelvin effect. What is inversion temperature? Refer chapter 5.21 on Page 5.44. 19. Derive Clausius-Clapeyron équation. What are the assumptions made in this equation? Refer chapter 5.22 on Page 5.49. 20. Using the Clapeyron equation, estimate the value of the enthalpy of vaporisation of refrigerant R-134a at 293K, and compare it with the tabulated value. Refer chapter 5.22 on Page 5.49. 21. What is meant by phase change process? Derive Clausius-Clapeyron equation for a phase change process. Give the significance of this equation. Refer chapter 5.23 on Page 5.52 for phase change process and chapter 5.22 on Page 5.49 for Clausius-Clapeyron equation. 22. Write down the Dalton's law of partial pressure and explain its importance. Refer chapter 5.26.2 on Page 5.75. 23. Explain the mole fraction and mass fraction and the relationship between them. Refer chapter 5.26.1 on Page 5.73. 24. State Amagat's Law and Dalton's Law. Refer chapter 5.26.3 on Page 5.77 for Amagat's Law and chapter 5.26.2 on Page 5.75 for Dalton's Law.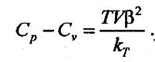

Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Gas Mixtures and Thermodynamic Relations | Engineering Thermodynamics - Solved Important 16 marks Long Questions
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
