Engineering Thermodynamics: Unit III: Availability and Applications of II Law
Solved Anna University Problems on Availability, Irreversibility and Thermodynamic Second Law Efficiency
Solved Anna University Problems on Availability, Irreversibility and Second Law Efficiency:
SOLVED ANNA UNIVERSITY PROBLEMS ON AVAILABILITY, IRREVERSIBILITY AND SECOND LAW EFFICIENCY AU Problem 3.21 A heat engine receives 800 kJ of heat from the reservoir at 1000 K and rejects 400 kJ at 400 K. If the surrounding is at 300 K, calculate the first and the second law efficiency and the relative efficiency of the heat engine. Given data: Q1 = 800 kJ T1 = 1000 K Q2 = 400 kJ T2 = 400 K To = 300 K Solution: Net work or work output, Wnet = Q1 – Q2 = 800 - 400 = 400 kJ AU Problem 3.22 200 m3 rigid tank initially contains atmospheric air at 100 kPa and 300 K and is to be used as a storage vessel for compressed air at 1 MPa and 300 K. Compressed air is to be supplied by a compressor that takes in atmospheric air at po = 100 kPa and To = 300 K. Determine the minimum work required for this process. Given data: V1 = V2 = 200 m3 (since the tank is rigid) p1 = 100 kPa = 100 kN/m2 T1 = 300 K p2 - 1 MPa = 1000 kN/m2 T2 = 300 K po = 100 kPa = 100 kN/m2 To = 300 K Solution: The minimum work required is to be calculated between exit of the compressor and ambient. AU Problem 3.23 One kg of air is contained in a piston cylinder assembly at 10 bar pressure and 500 K temperature. The piston moves outwards and the air expands to 2 bar pressure and 350 K temperature. Determine the maximum work obtainable. Assume the environmental conditions to be 1 bar and 290 K. Also make calculations for the availability in the initial and final states. Given data: m = 1 kg p1 = 10 bar T1 = 500 K p2 = 2 bar T2 = 350 K po = 1 bar To = 290 K Solution: AU Problem 3.24 Air expands through a turbine from 500 kPa, 520°C to 100 kPa, 300°C. During expansion 10 kJ/kg of heat is lost to the surroundings which is at 98 kPa, 20°C. Neglecting the kinetic and potential energy changes, determine per kg of air, (i) The decrease in availability, (ii) The maximum work, and (iii) The irreversibility. For air Cp = 1.005 kJ/kgK and h = CpT. Similar to AU Problem 3.23 on Page 3.65. [Ans:- Δψ = 260.8 kJ/kg, Wmax = Δψ = 260.8 kJ/kg, I = 38.9 kJ/kg] [Note: Work done during expansion, Wact = mCp (T1 - T2) = 1 × 1.005 (793 - 573) = 221.1 kJ/kg Since 10 kJ/kg of heat is lost to the surroundings, actual work done, Wact = 221.1 – 10 = 211.1 kJ/kg Irreversibility, I = Wmax - Wact = 260.8 - 211.1 = 49.7 kJ/kg ] AU Problem 3.25 Two kg of air at 500 kPa, 80°C expands adiabatically in a closed system until its volume is doubled and its temperature becomes equal to that of the surroundings which is at 100 kPa, 5°C. For this process determine the: (i) maximum work (ii) change in availability and (iii) irreversibility. Given data: m = 2 kg p1 = 500 kPa T1 = 80°C = 80 + 273 = 353 K V2 = 2V1 po = 100 kPa T2 = To = 5°C = 5 + 273 = 278 K Solution: Change in entropy, AU Problem 3.26 3 kg of air at 500 kPa, 90°C expands adiabatically in a closed system until its volume is doubled and its temperature becomes equal to the surroundings at 100 kPa and 10°C. Find the maximum work, change in availability and irreversibility. Similar to AU Problem 3.25 on Page 3.67. [Ans:- (i) Wmax = 189.46 kJ (ii) ψ1 – ψ2 = 126.9 kJ and (iii). = 17.14 kJ] AU Problem 3.27 5 kg of air at 550 K and 4 bar is enclosed in a closed vessel. (i) Determine the availability of the system if the surrounding pressure and temperature are 1 bar and 290 K. Similar to AU Problem 3.23 on Page 3.65. [Ans:- ψ1 = 576.7 kJ] (ii) If the air is cooled at constant pressure to the atmospheric temperature, determine the availability and effectiveness. Given data: m = 5 kg T1 = 550 K po = 1 bar T2 = To = 290 K Solution: For constant pressure cooling, Q = mCp (T1 - T2) = 5 × 1.005 × (550 - 290) = 1306.5 kJ Available energy of air during cooling, AU Problem 3.28 How much of the 100 kJ of thermal energy at 650 K can be converted to useful work? Assume the environment to be at 25°C. Given data: Q = 100 kJ T = 650 K To = 25°C = 273 + 25 = 298 K Solution: Available energy or useful energy, AU Problem 3.29 Air flows through an adiabatic compressor at 2 kg/s. The inlet conditions are 1 bar and 310 K and the exit conditions are 7 bar and 560 K. Compute the net rate of energy transfer and the irreversibility. Take To = 298 K. Given data: m = 2 kg/s p1 = 1 bar T1 = 310 K p2 = 7 bar T2 = 560 K To = 298 K Solution: AU Problem 3.30 Helium enters an actual turbine at 300 kPa, 300°C and expands to 100 kPa, 150°C. Heat transfer to atmosphere at 101.325 kPa, 25°C amounts to 7 kJ/kg. Calculate the entering stream availability, leaving stream availability and the maximum work. For helium, Cp = 5.2 kJ/kgK and molecular weight = 4.003 kg/kmol. Similar to AU Problem 3.23 on Page 3.65. AU Problem 3.31 In a passenger car, a lead storage battery is able to deliver 5.2 MJ of electrical energy. This energy available is used to start the car. Suppose we wish to use compressed air for doing an equivalent amount of work in starting the car. The compressed air is stored at 7 MPa, 25°C. Calculate the mass of the air and volume of tank required to have the compressed air having the same availability of 5.2 MJ. Take 101325 Pa and 298 K as atmospheric conditions. Given data: Total availability = 5.2 MJ = 5200 kJ p2 = p1 = 7 MPa = 7000 kPa T2 = T1 = 25°C = 25 + 273 = 298 K po = 101325 Pa = 101.325 kPa To = 298 K Solution: There is no end condition given. So, it is assumed with ambient conditions.
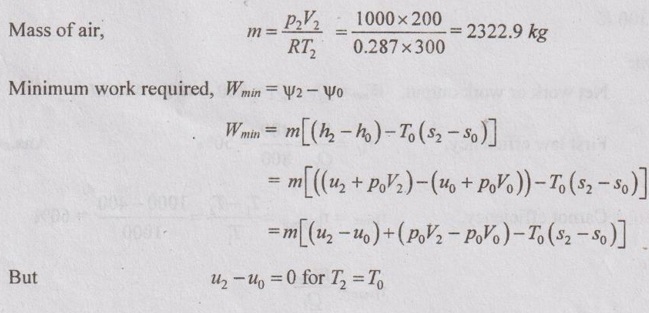 So, the above equation reduces to
So, the above equation reduces to


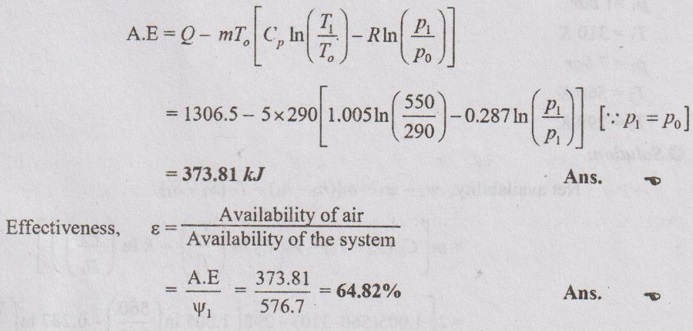
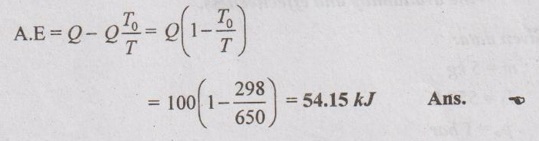

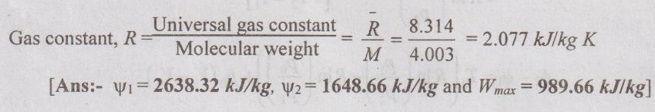

Engineering Thermodynamics: Unit III: Availability and Applications of II Law : Tag: : - Solved Anna University Problems on Availability, Irreversibility and Thermodynamic Second Law Efficiency
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
