Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Properties of Real Gas
The gas which does not obey the law of equation of state of ideal gas is known as real gas.
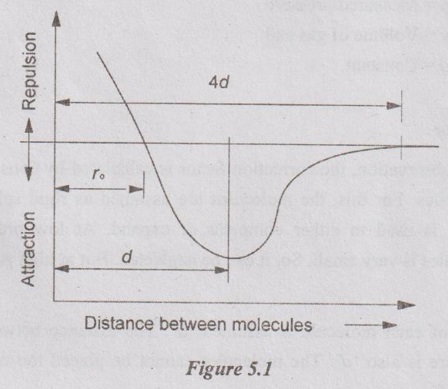
PROPERTIES OF REAL GAS The gas which does not obey the law of equation of state of ideal gas is known as real gas. In case of real gases, the specific heats constant vary with pressure and volume. The enthalpy and internal energy are the functions of pressure and temperature. So, the equations of entropy derived for ideal gas are not applicable for real gases. The new term is introduced in equation of state called compressibility factor. So, the equation of state for real gases becomes pv = ZRT where Z = Compressibility factor. In the first observation, the force of attraction between molecules at low pressure is noticed. First, the molecules are attracted each other at distance ro. When the distance between molecules increases, the attractive force will also increase and reach maximum as shown in Figure 5.1. When the distance reaches 4d or more, the attractive force almost will be negligible. where d is the diameter of the molecule. If the distance between molecules is less than ro, the molecules will repel each other. Again, the repulsive forces will increase as the distance still decreases. Let us, consider a vessel having real gases. In these gases, a molecule is exactly considered at the middle. If the molecule is not near the wall but surrounded by other molecules tightly, the force of attraction will be equal in all directions. It means, the resultant attraction force is zero. If the resultant force has some value, the molecule will be in such a way to hit the wall. This attraction force is directly proportional to the number of molecules per unit volume of the gas and frequency of collision with the wall. where p = Measured pressure v = Volume of gas and a = Constant. In the second observation, the correction factor is calculated by considering the volume occupied by molecules. For this, the molecules are assumed as rigid sphere and the space between molecules is used to either compress or expand. At low pressure, the volume occupied by molecules is very small. So, it can be neglected. But at high pressure, the volume cannot be ignored. The diameter of each molecule is assumed 'd'. The distance between two molecules from centre to centre is also 'd'. The molecules cannot be placed too close to each other. Then the distance 'd'. Eight molecules can be placed around each molecule. But the effective volume of molecules is 1. Intermolecular Forces

2. Shape Factor
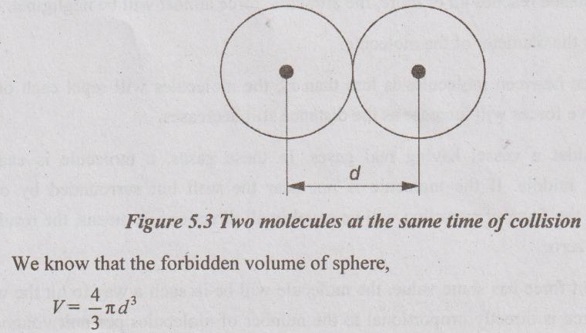
 Then, it is considered for four molecules around each molecule. It is known as correction factor. Generally, it is denoted by 'b'. So, the effective volume of the gas per unit mass is given by (v - b). To compensate the behaviour of the real gas molecules, these two corrections for pressure and volume are carried out.
Then, it is considered for four molecules around each molecule. It is known as correction factor. Generally, it is denoted by 'b'. So, the effective volume of the gas per unit mass is given by (v - b). To compensate the behaviour of the real gas molecules, these two corrections for pressure and volume are carried out.
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : - Properties of Real Gas
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
