Hydraulics and Pneumatics: Unit I: Fluid Power Priniciples and Hydraulic Pumps
properties of hydraulic fluids (characteristics of hydraulic fluids)
Density, Viscosity
The following properties of hydraulic fluids are of general importance to the study of hydraulic power systems.
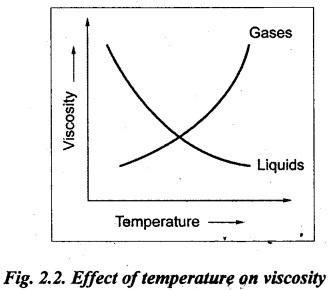
PROPERTIES OF HYDRAULIC FLUIDS (CHARACTERISTICS OF HYDRAULIC FLUIDS) The following properties of hydraulic fluids are of general importance to the study of hydraulic power systems. The density of a fluid is that quantity of matter contained in unit volume of the substance. The density can be expressed in three different ways as discussed below. 1. Mass Density • Definition: Mass density (p), also known as specific mass or simply 'density', is defined as the mass of the fluid per unit volume. • Formula: • Units: kilograms per cubic metre (kg/m3) • Dimensions: ML-3 • Typical values: At atmospheric pressure (i.e., p = 1.013 × 105 N/m2) and temperature (i.e., T = 288.15 K), ρwater = 1000 kg/m3, and ρair = 1.23 kg/m3. 2. Weight Density • Definition: Weight density (w), also known as specific weight, is defined as the weight per unit volume. • Formula: • Relationship between w and ρ: Since weight is dependent on gravitational attraction, the specific weight will vary from point to point, according to the local value of gravitational acceleration 'g'. The relationship between w and ρ can be obtained from Newton's second law. That is, • Units: newtons per cubic metre (N/m3). • Dimensions: ML-2 T-2 • Typical values: wwater = 9.81 × 103 N/m3 ; wair = 12.07 N/m3 3. Specific Gravity • Definition: Specific gravity (S), also known as relative density, is defined as the ratio of mass (or weight) density of a fluid to mass (or weight) density of a standard fluid. • Formula: • For liquids, the standard mass density chosen is the maximum density of water (which occurs at 4°C at atmospheric pressure). For gases, the standard density may be that of air or hydrogen at a specified temperature and pressure. • Units: No units (⸪ Relative density is a ratio of two quantities of the same kind, it is a pure number having no units). • Dimensions : Since it is a pure number, its dimensions are M0L0T0 = 1. • Typical values: Swater = 1.0; Soil = 0.9. 4. Specific Volume • Definition: Specific volume (v) is defined as the reciprocal of mass density. • Formula: • Units: cubic metre per kilograms (m3/kg). • Dimensions: M-1 L3 • Typical values: Vwater = 0.001 m3/kg; Vair = 0.813 m3/kg • Concept: Viscosity is the most important property of the fluid. Viscosity is the measure of the ability of a fluid to flow. It is the measure of the fluid's internal resistance to shear or flow at a definite temperature and pressure. • Definition: Viscosity is defined as the shearing force required to move two plane surfaces relative to one another with a film of fluid between them. • Newton's law of viscosity: This law states that the shear stress (τ) on a fluid element layer is directly proportional to the rate of shear strain. The constant of proportionality is called the coefficient of viscosity. • Viscosity may be defined/expressed in number of different ways such as: 1. Absolute viscosity, and 2. Kinematic viscosity. 1. Absolute Viscosity • Definition : Absolute viscosity (μ), also known as coefficient of dynamic viscosity, is defined as the shear stress (t) required to produce unit rate of shear strain (du/dy). • Formula: Rearranging equation (2.6), we get • Units: newtons seconds per square metre (N.s/m2) or kilograms per metre per second (kg/m/s). [But it should be noted that the coefficient of viscosity is often measured in poise (P); 10 P = 1 kg/m/s.] • Dimensions : ML-1T-1 • Typical values: μwater = 1.14 × 10-3 kg/m/s (or 0.0114 P); μair == 1.78 × 10-5 kg/m/s. 2. Kinematic Viscosity • Definition : Kinematic viscosity (v) is defined as the ratio of dynamic viscosity to mass density. • Formula: • Units: square metres per second (m2/s). [But it should be noted that kinematic viscosity is often measured in strokes (St); 104 St = 1 m2/s.] • Dimensions: L2T-1 ́ • Typical values: Vwater = 1.14 × 10-6 m2/s (or 0.0114 St); Vair = 1.46 × 10-5 m2/s. Note 1. The instrument most oftenly used by engineers and technicians to measure the viscosity of liquids (oils) is the Saybolt viscometer or viscosimeter. 2. The measured viscosity is usually expressed as Seconds Saybolt Universal (SSU) or Saybold Universal. Seconds (SUS), at certain temperature. The majority of hydraulic systems operate most efficiently with fluids having the viscosity ranges: 135 to 165 SSU; 185 to 230 SSU; and 275 to 315 SSU. 3. The empirical relationship between the viscosity in SUS and CS can be given as below: where v = Kinematic viscosity in cS, and t = Time measured in SUS or in simply seconds. 4. SAE viscosity grade: The commercial classification of oils established by the Society of Automotive Engineers (SAE) relates viscosity range to a SAE number. For example, SAE 20 oil has a viscosity of 120 to 185 SSU/SUS at 130°F and SAE 30 oil has a viscosity of 185 to 225 SSU/SUS at the same temperature. • Significance of viscosity: Various components within a system may have different requirements such as low or high viscosity. 1. If the fluid is too thick (i.e., viscosity is higher than the recommended), then the following behaviours will result in the system : (i) Increases in load and hence increased power loss. (ii) High operating temperatures because there will be internal friction. (iii) Excessive wear of parts.. (iv) Reduces internal leakage. 2. If the fluid is too thin (i.e., viscosity is lesser than the recommended), then the following behaviours will happen in the system : (i) Less internal friction. (ii) Smaller pressure losses in pipes and valves. (iii) Increase in control action and component response. (iv) Increases internal and external leakage. (v) Cannot lubricate properly. (vi) Lead to unnecessary rapid wear of moving parts. Thus a compromise in viscosity requirements must be made. • Effect of temperature on viscosity of liquids : Temperature has an adverse effect on the viscosity of the hydraulic oils. The viscosity of the liquids decreases with increase in temperature (Fig.2.2). This is because of the shear stress in the liquids (due to the intermolecular cohesion) decreases decreases increase of temperature. • Effect of pressure on viscosity of liquids: The viscosity of the liquids is not appreciably affected by the changes in pressure. However, high pressures will affect the viscosity of the liquids. During high pressures, the energy required for the relative movement of the molecules is increased. Therefore, the viscosity of liquids increases with increasing pressure. Note • Effect of temperature on viscosity of gases: If the temperature of a gas increases, the molecular interchange will increase. Therefore the viscosity of a gas increases with increase in temperature (Fig.2.2). • Effect of pressure on viscosity of gases: Over the normal ranges of pressures, the viscosity of a gas is found to be independent of pressure. But it is affected by very high pressures. 3. Viscosity Index • Definition: The viscosity index (V.I) of a liquid is a number indicating the effect of a change in temperature on viscosity. The V.I is expressed in some arbitrary numerical values. • Concept: The viscosity of high V.I oils is less sensitive than the viscosity of low V.I oils. In other words, the rate of change in viscosity with changes in temperature is relatively less with high V.I oils than with low V.I oils. • Formula: The V.I of any hydraulic fluid can be calculated by the following equation : where L = Viscosity in SUS of 0-VI oil at 100°F, U = Viscosity in SUS of unknown-VI oil at 100°F, and H = Viscosity in SUS of 100-VI oil at 100°F. 4. Cohesion and Adhesion • Cohesion: Cohesion means intermolecular attraction between molecules of the same liquid. It enables a liquid to resist small amount of tensile stresses. Cohesion is an tendency of the liquid to remain as one assemblage of particles. • Adhesion: Adhesion means the attraction between the molecules of a liquid and the molecules of a solid boundary surface in contact with the liquid. This property enables the liquid to stick to another body. 5. Surface Tension • A liquid, being unable to expand freely, will form an interface with a second liquid or gas. Molecules deep within the liquid repel each other because of their close packing. Molecules at the surface are less dense and attract each other. When half of their' neighbours are missing, the mechanical effect is that the surface is in tension. This phenomenon is known as surface tension. • The effect of surface tension is to reduce the surface of a free body of liquid to a minimum. Surface tension effects can be reduced by the addition of detergents. 6. Capillarity • Capillarity is a phenomenon by which a liquid (depending upon its specific gravity) rises into a thin glass tube above or below its general level. • This phenomenon is due to the combined effect of cohesion and adhesion of liquid particles. • Capillarity action is a serious source of error in reading liquid levels in fine-gauge tubes. To minimise errors due to capillarity, gauge glasses having a larger diameter may be used to read the level of liquids. 7. Vapour Pressure • The pressure exerted by a vapour which is in equilibrium with the liquid is known as vapour pressure. • The vapour pressure will increase with the increase in temperature. • Boiling of liquids will occur when the vapour pressure is equal to the pressure above the liquid. By reducing the pressure, boiling can be made to occur at temperatures well below the boiling point at atmospheric pressure. For example, if the pressure is reduced to 0.2 bar (0.2 atm), water will boil at a temperature of 60°C. 8. Cavitation • When the liquid pressure is dropped below the vapour pressure due to a flow phenomenon, then there will be local boiling and a cloud of vapour bubbles will form. This phenomenon is known as cavitation. • Cavitation causes serious damage in almost any component in a hydraulic system. Cavitation can affect the performance of hydraulic machinery such as pumps, turbines, and propellers. 9. Compressibility • All fluids are compressible to some extent. Compressibility of a liquid causes the liquid to act much like a stiff spring. • The coefficient of compressibility is the fractional change in a unit volume of liquid per unit change of pressure. Mathematically, where ∆V = Change in volume, V = Original volume, and ∆P = Change in pressure. • Generally it is desirable to have the hydraulic fluid which has the minimum compressibility. That is, the fluid with minimum compressibility will be more stiffer. 10. Bulk Modulus • Bulk modulus is the reciprocal of compressibility. • It is the measure of compressibility of a fluid. Mathematically, • The higher the bulk modulus, the less elastic or more stiffer the liquid. Usually high bulk modulus values are desirable since they result in more stable and less elastic systems. 11. Cloud Point • Definition: Cloud point is defined as the temperature at which wax or other dissolved solids begin to crystallise and become noticeable when liquids are chilled under specified conditions. • Significance: The cloud point is of interest for use at low temperatures. Many liquids have no cloud point. 12. Pour Point • Definition : The temperature at which an oil will congeal is known as the pour point. In other words, the pour point of an oil indicates the temperature below which the oil will not flow freely. • Significance: This property is very important for hydraulic systems required to operate in extremely cold weather. • Chemical additives can be used to lower the pour point. • Any fluid used for hydraulic purposes should have a pour point well below its minimum operating temperature. 13. Flash and Fire Points • Flash point: The flash point is the minimum temperaturẻ at which sufficient liquid is vapourized under specified conditions to create a mixture that will burn if ignited. As the name 'flash' indicates, burning at this point is only an instantaneous flash. • A high flash point is desirable because it indicates good resistance to combustion and a low degree of evaporation at normal or working temperatures. Congeal = (Cause to) become semi-solid by cooling. • Fire point: The fire point is the minimum temperature at which sufficient vapour is continuously generated to sustain combustion. • Significance of flash and fire points : Flash and fire points indicate the temperature at which the liquid begins to volatilize. A lower flash or fire point is an indication that the liquid has become contaminated with a more volatile product (such as a fuel). • Autogenous ignition temperature (AIT): It is the temperature at which ignition occurs spontaneously. 14. Demulsibility • Definition: The property of hydraulic fluid to separate rapidly and completely from moisture and to resist emulsification is known as demulsibility. • Significance: This property is considerably significant because the operation of many hydraulic systems are conductive to the forming of moisture or of stable water-in-oil emulsions. • Highly refined, hydraulic oils have excellent demulsibility characteristics. Also it is found that used or contaminated liquids are more likely to emulsify with water than new liquids. 15. Oxidation Stability • Definition : Oxidation stability is defined as the ability of a liquid to resist reaction with oxygen or oxygen-containing compounds. • Significance: This is an important property that affects storage life of hydraulic fluids and performance life of hydraulic fluids used in open systems. Note In addition to the property of oxidation stability, other noteworthy important chemical properties include: (a) Chemical stability : It refers to the ability of hydraulic fluids to be chemically stable under the imposed conditions. (b) Thermal stability: It refers to the ability of hydraulic fluids to resist decomposition by temperature only. (c) Hydraulic stability : It refers to the ability of hydraulic fluids to resist reaction with water. 16. Film Strength, Oiliness and Lubricity • The three properties film strength, oiliness and lubricity are pertained to lubricating characteristics of liquids. These properties can be improved with the use of additives. • Film strength: Film strength is the ability of a surface film to resist rupture by the penetration of asperities during sliding or rolling. Generally the higher viscous fluids have higher film strengths. • Oiliness: Oiliness is the property of a liquid which causes films of two liquids of identical viscosity to exhibit different coefficients of friction. • Lubricity: Lubricity is the ability of a liquid to impart low friction under boundary conditions. 17. Compatibility • Definition: Compatibility is the ability of the hydraulic fluid to be compatible with the system. • That is, the hydraulic fluid should be compatible with the materials used in the hydraulic system, including metals, plastics, surface coatings, elastomers, lubricants, and other hydraulic fluids. If the hydraulic fluid in any way attacks, destroys, dissolves, or changes any part of the hydraulic system, the system may become inoperable. Similarly, any changes in the hydraulic fluid caused by interaction with the system materials can also cause system malfunction. Therefore, any hydraulic fluid must be compatible with the systems. 18. Volatility • The volatility of a liquid describes the degree and rate at which it will vapourize under given conditions of temperature and pressure. • It is desirable that a hydraulic fluid have low volatility. 19. Corrosiveness • The corrosiveness of a hydraulic fluid refers to its tendency to promote or encourage corrosion in a hydraulic system. • Obviously, it is desirable to maintain the corrosiveness of a hydraul fluid at as low level as possible. For this purpose, rust- and corrosion-preventive synthetic chemicals are usually added with hydraulic fluids. 20. Neutralization Number • The term 'Neutralization number' is used to express an acidity or alkalinity of lubricating liquids and hydraulic fluids. • Definition: The neutralization number is defined as the number of milligrams of potassium hydroxide required to neutralize all the acids present in one gram of the sample.1. Density


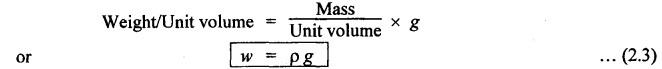
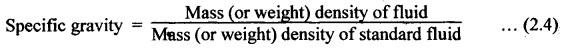
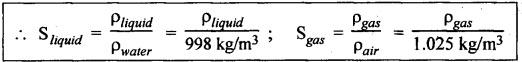
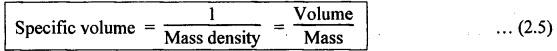
2. Viscosity
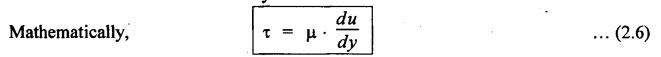
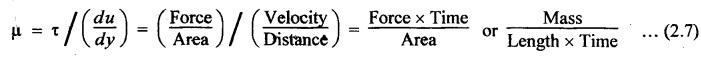

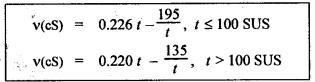



Hydraulics and Pneumatics: Unit I: Fluid Power Priniciples and Hydraulic Pumps : Tag: : Density, Viscosity - properties of hydraulic fluids (characteristics of hydraulic fluids)
Related Topics
Related Subjects
Hydraulics and Pneumatics
ME3492 4th semester Mechanical Dept | 2021 Regulation | 4th Semester Mechanical Dept 2021 Regulation