Hydraulics and Pneumatics: Unit IV: Pneumatic and Electro Pneumatic Systems
properties of air
Pneumatic and Electro Pneumatic Systems - Hydraulics and Pneumatics
Actually speaking, air is a mixture of gases. Air is invisible, colourless, odourless, and tasteless.
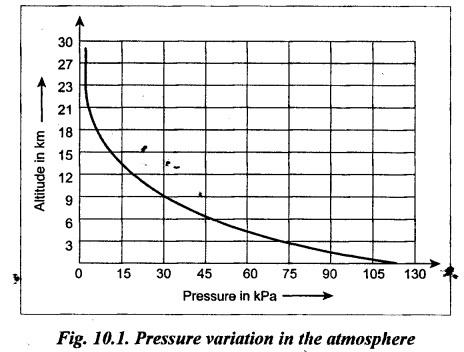
PROPERTIES OF AIR • Actually speaking, air is a mixture of gases. Air is invisible, colourless, odourless, and tasteless. • Composition: The main constituents of air by volume are 78% nitrogen, 21% oxygen, and 1% other gases such as argon and carbon dioxide. • The gaseous layer of air around the earth is known as atmosphere. • Atmospheric pressure: The air surrounding the earth exerts a pressure on the earth's surface. The pressure prevailing directly on the earth's surface is known as atmospheric pressure. • The atmospheric pressure is also referred to as reference pressure. Normally it considers the sea level as its reference point. • The atmospheric pressure may be calculated from the fundamental principle of barometer which states that the barometer reads the pressure due to the height of mercury (Hg) in the tube and its weight. where ρ = Density of Hg = 13600 kg/m3, g = Acceleration due to gravity = 9.81 m/s2, and h = Height of Hg column = 760 mm of Hg at normal sea level. Substituting the above values in equation (10.1), we get Atmospheric pressure = 13600 × 9.81 × 0.76 = 1,01,396 N/m2 = 1.013 bar But for easy and simple calculation, we take the atmospheric pressure as 1 bar. • Absolute and gauge pressure: As discussed in Section 3.3.5, the relationship between atmospheric, absolute, and gauge pressures can be given as below: • Pressure variation with altitude: The atmospheric pressure decreases with the increase in altitude, as shown in Fig.10.1. From Fig.10.1, it may be noted that the pressure varies linearly upto an altitude of 6 km and after that the pressure drops about 11 kPa per km change in altitude. • Standard air: Standard air is sea-level air having a temperature of 20°C, pressure of 1.013 bar, and a relative humidity of 36%. Usually the above valves of standard air are used while making pneumatic system calculations. We know that, Boyle's law, Charles' law, Gay-Lussac's law and the general gas law are called the 'perfect gas laws' because they were derived on the basis of a perfect gas. The air behaves like a perfect gas or an ideal gas with very insignificant deviation from the perfect gas. Therefore all the perfect gas laws are equally applicable to air. 1. Boyle's Law • Boyle's law states that if temperature remains constant, the pressure of a confined mass of gas will vary inversely with its volume. • The term relative humidity can be defined as the ratio of the partial pressure of water vapour in a given volume of mixture to the partial pressure of water vapour when the same volume of mixture is saturated at the same temperature. 2. Charles' Law • Charles' law states that pressure remaining constant, the volume of a given mass of gas will vary directly as its absolute temperature. 3. Gay-Lussac's Law • Gay-Lussac's law states that volume remaining constant, the pressure of a confined mass of gas will vary directly as its absolute temperature. 4. General Gas Law • Boyle's, Charles', and Gas-Lussac's laws can be combined to obtain the general gas law and is given by, Note In the perfect gas law, the P and T represents absolute pressure and absolute temperature (in °K) respectively. The properties, discussed in Section 2.4, such as density, specific volume, viscosity, compressibility, bulk modulus, etc., are also equally applicable to the air.1. What is the Composition of Air?
2. Pressure Relationship

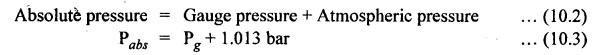
3. Perfect Gas Laws

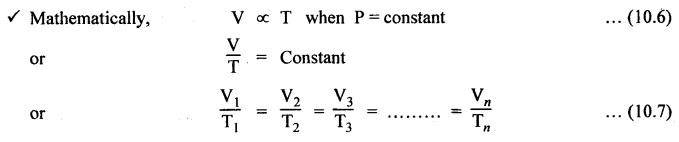


4. Other Properties of Air
Hydraulics and Pneumatics: Unit IV: Pneumatic and Electro Pneumatic Systems : Tag: : Pneumatic and Electro Pneumatic Systems - Hydraulics and Pneumatics - properties of air
Related Topics
Related Subjects
Hydraulics and Pneumatics
ME3492 4th semester Mechanical Dept | 2021 Regulation | 4th Semester Mechanical Dept 2021 Regulation