Engineering Thermodynamics: Unit III: Availability and Applications of II Law
Entropy Change of Ideal Gas
Consider an ideal gas heated from state 1 to state 2 and thus, the temperature is increased from T1 to T2 as shown in Figure 3.1.
Unit-3 AVAILABILITY AND APPLICATIONS OF II LAW Ideal gases undergoing different processes - principle of increase in entropy. Applications of II Law. High and low- grade energy. Availability and Irreversibility for open and closed system processes - I and II law Efficiency 1. ENTROPY CHANGE OF IDEAL GAS Consider an ideal gas heated from state 1 to state 2 and thus, the temperature is increased from T1 to T2 as shown in Figure 3.1. During the heating process, there should be some change in entropy in the gas. If a change in heat transfer dQ is considered to the gas at an absolute temperature T, then the change in entropy is given by ds = dQ / T. 1. Expression for Change in Entropy of Ideal Gas (i) In terms of temperature and volume: By the law of conservation of energy or first law of thermodynamics, Dividing equation (3.1) throughout by 'T', Integrating above equation from state 1 to state 2, (ii) In terms of pressure and temperature: By gas equation, Substituting (3.3) in (3.2) change in entropy, Also, it can be written as (iii) In terms of pressure and volume by gas equation: Substituting this value in equation (3.2) change in entropy,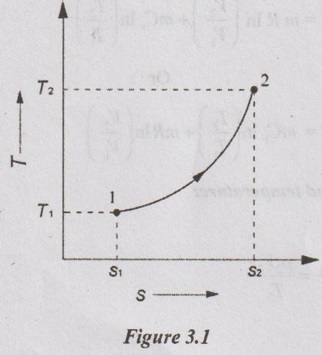

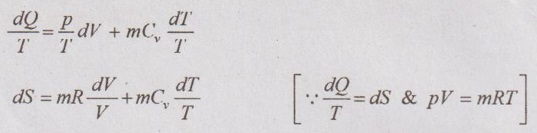
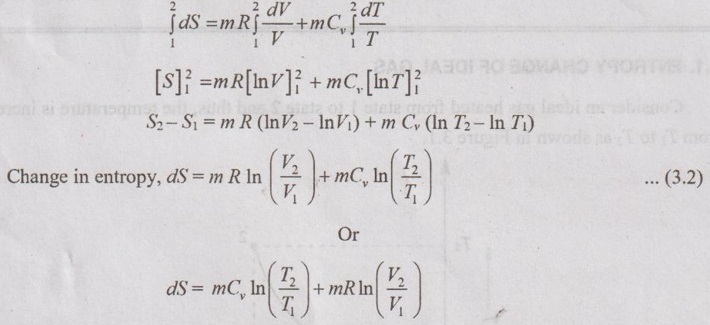

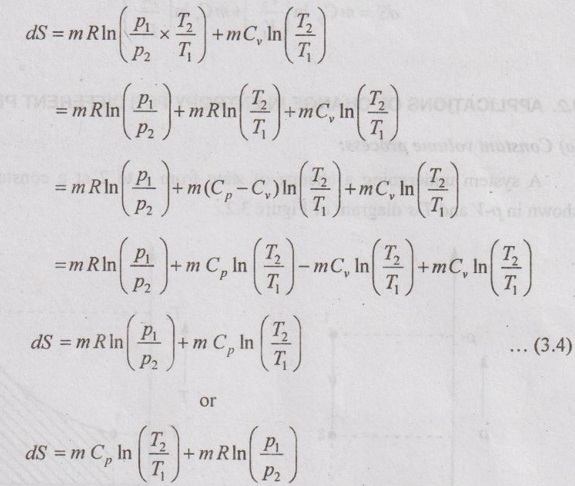
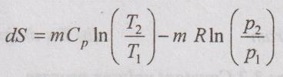


Engineering Thermodynamics: Unit III: Availability and Applications of II Law : Tag: : - Entropy Change of Ideal Gas
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
