Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Energy Equations
Internal Energy, Enthalpy Relations | Thermodynamics
Mainly, the energy equations are internal energy, enthalpy and entropy.
ENERGY EQUATIONS Mainly, the energy equations are internal energy, enthalpy and entropy. We know that the internal energy du = Tds - pdv Let the entropy (s) is the function of temperature (T) and specific volume (v). From equation (5.66), the internal energy in terms of measurable properties such as p, v, T and Cv can be determined. We know that from first law of thermodynamics Enthalpy, dh = Tds + vdp [from equation (5.57)] Let the entropy (s) is the function of temperature (T) and specific volume (v). i.e. s = f(T, p) Substituting ds value in dh equation, The above equation (5.68) appears in the form of dh = CpdT + Constant So, Cp can be written as From Maxwell equations, From equation (5.70), the enthalpy in terms of measurable properties such as p, v, T and Cp can be calculated.1. Internal Energy

2. Enthalpy Relations
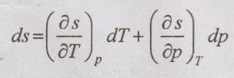
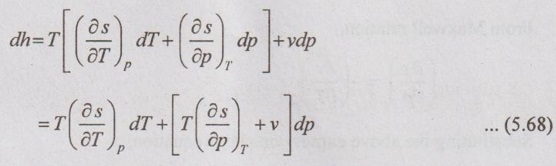

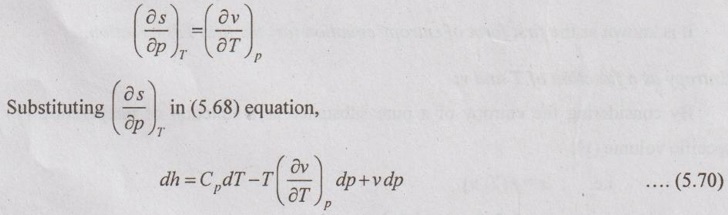
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Internal Energy, Enthalpy Relations | Thermodynamics - Energy Equations
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
