Engineering Physics: Unit IV: Basic Quantum Mechanics
Electrons (particles) and Matter Waves - (Concept of Matter Waves)
Particle nature of matter is very well established. Now it is known that matter is composed of atoms, electrons, protons and neutrons.
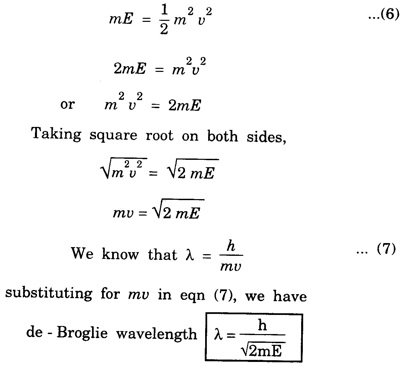
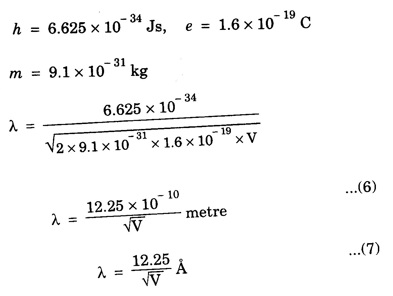
ELECTRONS (Particles) AND MATTER WAVES - (Concept of Matter Waves) • Particle nature of matter is very well established. Now it is known that matter is composed of atoms, electrons, protons and neutrons. They are the building blocks of all types of atoms. • Electromagnetic theory and quantum theory established wave-particle duality of radiation. But inspite of the success of this duality of radiation, the postulates of Bohr's theory of atomic structure remained unexplained for a long time. • On this background, in 1924 De Broglie extended the idea of dual nature of radiation to matter and proposed that matter possesses particle as well as wave characteristics. • He believed that motion of electron within an atom is guided by a peculiar kind of waves called 'Pilot waves'. • While introducing the concept of matter waves, De Broglie was guided by wave - particle duality of radiation and the way in which nature manifests herself. The concept of wave nature of matter is developed from the dual character of radiation which sometimes behaves as a wave and at other times as a particle. Louis de - Broglie proposed a very bold and novel suggestion that like light radiation, matter or material particle also posseses dual (two) characteristics i.e., particle - like and wave - like. The moving particles of matter such as electrons, protons, neutrons, atoms or molecules exhibit the wave nature in addition to particle nature. According to de - Broglie hypothesis, a moving particle is always associated with waves. (Fig. 6.5) • Waves and particles are the only two modes through which energy can propagate in nature. • Our universe is fully composed of light radiation and matter. • Since nature loves symmetry, matter and waves must be symmetric. • If electromagnetic radiation like light, X - rays can act like a wave and a particle, then material particles (electrons, protons etc) should also act like a particle and a wave. • Every moving particle is always associated with a wave. The waves associated with the matter particles are called matter waves or de - Broglie waves. From Planck's theory, the energy of a photon (particle nature) of frequency v is given by E = hv …...(1) According to Einstein's mass - energy relation E = mc2 …...(2) where m - mass of the photon c - velocity of the photon. Equating (1) and (2), we get hv = mc2 …...(3) Since mc = p momentum of a photon, The wavelength of de - Broglie wave associated with any moving particle of mass m with velocity v (momentum p = mv) is given by This equation (5) is known as de - Broglie's wave equation. We know that the kinetic energy E = 1/2 mv2 Multiplying by m on both sides we get, de - Broglie's wavelength in terms of accelerating potential associated with electrons When an electron of charge e is accelerated by a potential difference of V volts, then the electron gains a velocity v and hence, Workdone on the electron = eV ... (1) This workdone is converted into the kinetic energy of the electron as 1/2 mv2 Workdone = kinetic energy Multiply by m on both sides, we have Taking square root on both sides, we get From the de - Broglie's concept, the wavelength associated with any moving particle is given by Substituting eqn (3) in eqn (4), we have Substituting the given values, we have 1. If the mass of the particle is smaller, then the wavelength associated with that particle is longer. 2. If the velocity of the particle is small, then the wavelength associated with that particle is longer. 3. If v = 0, then 4. These waves do not depend on the charge of the particles. This shows that these waves are not electromagnetic waves. 5. The velocity of de - Broglie's waves is not constant since it depends on the velocity of the material particle.de - Broglie's Hypothesis
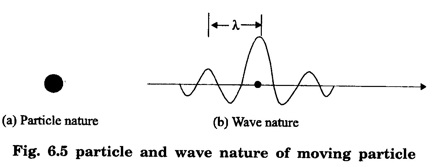
de - Broglie waves and its wavelength



de - Broglie wavelength in terms of energy

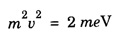
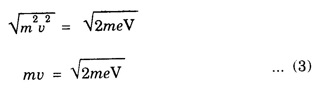


Properties of Matter Waves
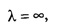 i.e., the wave becomes indeterminate and if
i.e., the wave becomes indeterminate and if ![]() then λ = 0. This indicates that de - Broglie waves are generated by the motion of particles.
then λ = 0. This indicates that de - Broglie waves are generated by the motion of particles.
Engineering Physics: Unit IV: Basic Quantum Mechanics : Tag: : - Electrons (particles) and Matter Waves - (Concept of Matter Waves)
Related Topics
Related Subjects
Engineering Physics
PH3151 1st semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
