Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Difference between specific heat capacities
Thermodynamics
The first Tds equation is given by
DIFFERENCE BETWEEN SPECIFIC HEAT CAPACITIES The first Tds equation is given by Similarly, the second Tds equation is given by Equating these two Tds equations, Comparing the coefficients of dv and dp in equation (5.79) and (5.80), it can be written as These two equations have common term of (Cp - Cv), the expression can be written as The equation (5.81) is the first form of (Cp - Cv) equation. The same (Cp - Cv) equation can be obtained in other form also shown below. We know that there are two specific heats of gas, i.e. Cp and Cv can experimentally be measured. From the definition of specific heats, Let, the entropy (s) is a function of temperature (T) and specific volume (v). From the equation (5.88), the difference between the two specific heats Cp and Cv is determined in terms of measurable properties p, v and T.

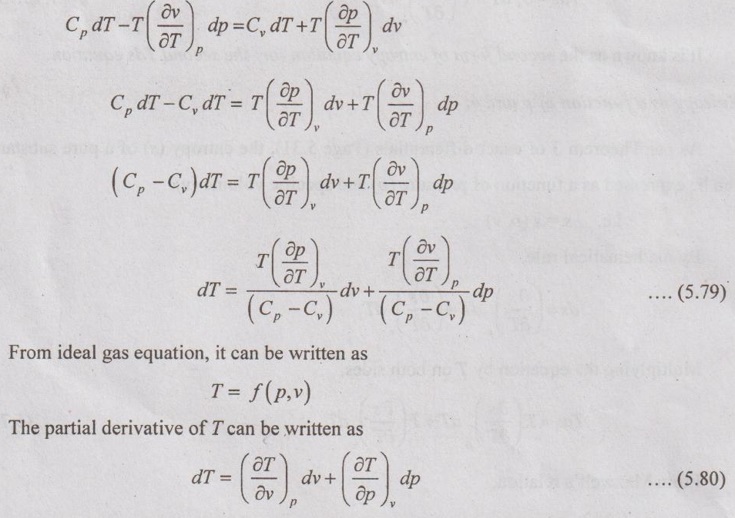
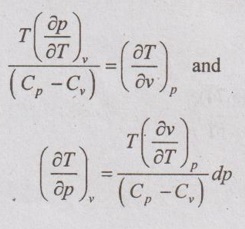



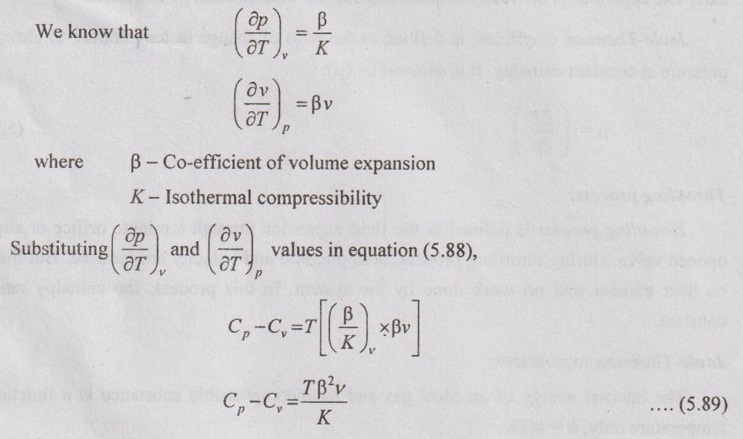
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Thermodynamics - Difference between specific heat capacities
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
