Engineering Physics: Unit IV: Basic Quantum Mechanics
Correspondence Principle
Statement, Definition, Equation, Proof, Significance | Quantum Mechanics
In 1932 Niels Bohr proposed a correspondence principle.
CORRESPONDENCE PRINCIPLE In 1932 Niels Bohr proposed a correspondence principle. Classical mechanics deals with the Laws of Physics governing macroscopic bodies. The Laws of Quantum Physics are applicable to the behaviour of microscope particles viz., atoms, nuclei etc. Bohr's correspondence principle bridges the gap between the classical mechanics and quantum mechanics. It removes the apparent discontinuity between the two. According to the correspondence principle, a system in a state of higher quantum number (higher excitation) is governed by the laws of classical mechanics. It does not mean that the laws of classical mechanics are applicable to all large systems. For systems at very low temperatures (near zero degree Kelvin) or in very low state of quantum numbers (low excitation), laws of quantum mechanics are applicable. The example are He3, He II, super conductors etc. The principle states that for large quantum numbers, quantum physics gives the same results as those of classical physics. According to classical electro-magnetic theory, an electron revolving in a circular orbit radiates electro-magnetic waves having a frequency equal to the frequency of revolution, including harmonics which are integral multiples of that frequency. The velocity of an electron revolving round the nucleus in an orbit of radius r is given by Taking root on both sides, we have The frequency of revolution Substituting for v we have. Substituting for r, we have According to Bohr's theory of the hydrogen atom When the quantum number involved are large i.e. n1 = n and n2 = n + 1 where n >> 1 As n >> 1, so neglecting 1 as compared to n and 2n, we get Comparing equations (4) and (5) we find that the classical orbital frequency and frequency of radiation emitted as calculated on the basis of quantum theory have the same value. Hence both classical and quantum theories of the hydrogen atom make identical predictions in the case of very large quantum numbers. In fact 'the greater the quantum number, the closer quantum physics approaches classical physics'. The correspondence principle has proved to be of great use in the computation of the intensity, polarisation and coherence of spectral radiation. It has also been helpful in the formulation of 'selection rules'.Statement
Proof

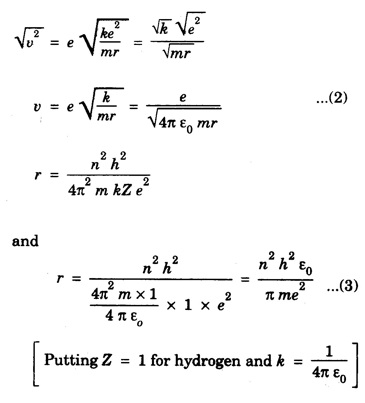

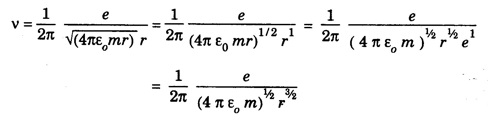
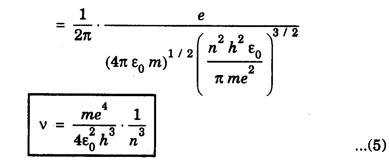
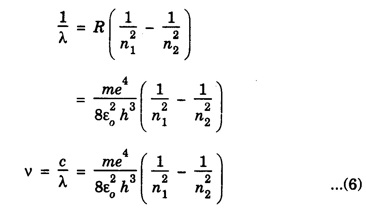
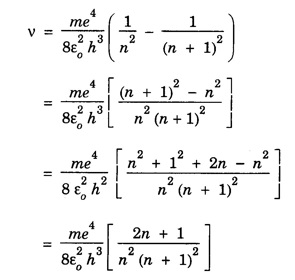
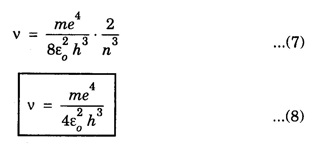
Significance of correspondence principle
Engineering Physics: Unit IV: Basic Quantum Mechanics : Tag: : Statement, Definition, Equation, Proof, Significance | Quantum Mechanics - Correspondence Principle
Related Topics
Related Subjects
Engineering Physics
PH3151 1st semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
