Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations
Clausius Clapeyron Equation
Thermodynamics
Clapeyron equation involves the relationship between saturation pressure and saturation temperature.
CLAUSIUS CLAPEYRON EQUATION Clapeyron equation involves the relationship between saturation pressure and saturation temperature. Both enthalpy of evaporation and specific volume of the two phases are also involved. This equation provides a basis for the calculation of properties in a two phase region. It gives the slope of a curve separating the two phases in p-T diagram. When the phase is changing from saturated liquid to saturated vapour, the temperature remains constant. So, ds equation reduces to Integrating the above equation between saturated liquid (f) and saturated vapour (g), This equation is known as Clausius-Clapeyron or Clapeyron equation. The above equation is valid for the change from a solid to liquid and solid to vapour. At very low pressures, vg = vfg is assumed. So, it approaches pv = RT. Therefore, the above equation (5.103) becomes We know that the equation of state for ideal gas Substituting vg in equation (5.104), it becomes Integrating the above equation, The equation (5.105) is also known as Clausius-Clapeyron equation.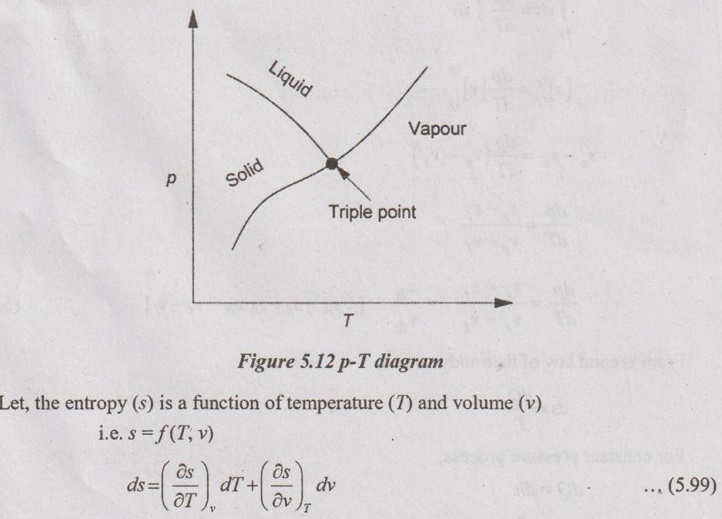


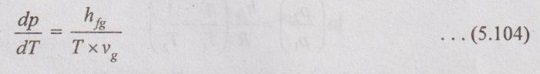
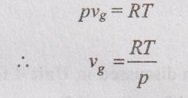
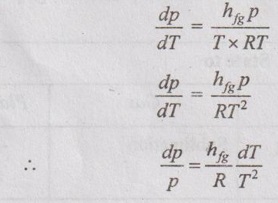
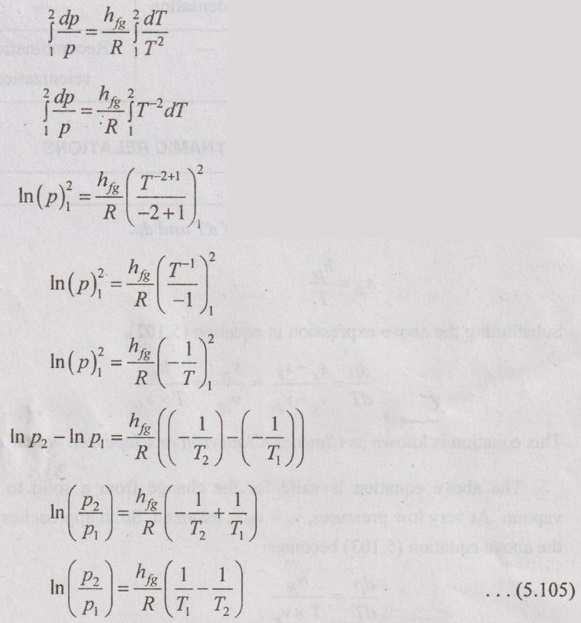
Engineering Thermodynamics: Unit V: Gas Mixtures and Thermodynamic Relations : Tag: : Thermodynamics - Clausius Clapeyron Equation
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
