Engineering Thermodynamics: Unit III: Availability and Applications of II Law
Available Energy and Unavailable Energy
Thermodynamics
The portion of the energy supplied as heat which can be converted into useful work by a reversible engine.
AVAILABLE ENERGY AND UNAVAILABLE ENERGY The portion of the energy supplied as heat which can be converted into useful work by a reversible engine. Consider a Carnot engine operating between the temperature limits T1 and T2 as shown in Figure 3.15. According to the second law of thermodynamics, the area of 1-2- 3-4 is called useful energy. But, the area of 2-3-5-6 refers the energy losses to the surroundings due to friction (uncontrollable loss). Our main aim is to reduce energy losses to the surroundings. Heat rejection or energy loss can be reduced by decreasing the temperature T2 to To (temperature of surroundings). By doing this, useful energy will increase with decrease in energy loss. We already know that no engine can have higher efficiency than Carnot engine. [One of the corollaries of second law]. According to this, The portion of the energy supplied as heat which cannot be converted into work due to friction is called unavailable energy. Unavailable energy, U.A.E = Total heat energy - Available energy = Q - A.E = Q - [Q - To ΔS] = To ΔS [Hint:- Unavailable energy = Loss in available energy (or) Irreversibility (or) Principle of entropy generation] Decrease in available energy through a finite temperature difference: Consider a reversible heat engine operating between T1 and To. Heat transfer, Q1 = T1 ΔS Heat transfer, Q2 = To ΔS Work output, W = Available energy W = T1 ΔS - To ΔS = (T1 - To) ΔS Assuming that heat Q1 is supplied to the engine. The temperature will be T1' when this heat reaches heat engine, not T1. But, the reservoir will be at the same temperature T1. Therefore, entropy due to T1' will increase from ΔS to ΔS". According to reversible heat engine concept, Q1' = T1 ΔS = T1' ΔS' Heat rejected by the heat engine will be Q2' not Q2. ⸫ Q2' = To ΔS' Actual work done by the heat engine, W' = Q1' – Q2' = T1' ΔS' - To ΔS' = (T1' - To) ΔS' We know that the work output, W = (T1 - To) ΔS ⸫ The actual work output of heat engine will be less because Q2' > Q2 ⸫ Decrease in available energy, W – W = Q2' - Q2 If the temperature difference between T1 and T1' is greater, the decrease in available energy will be more. Thus, there is a decrease in available energy when heat is transferred through a finite temperature difference.Available energy (A.E):
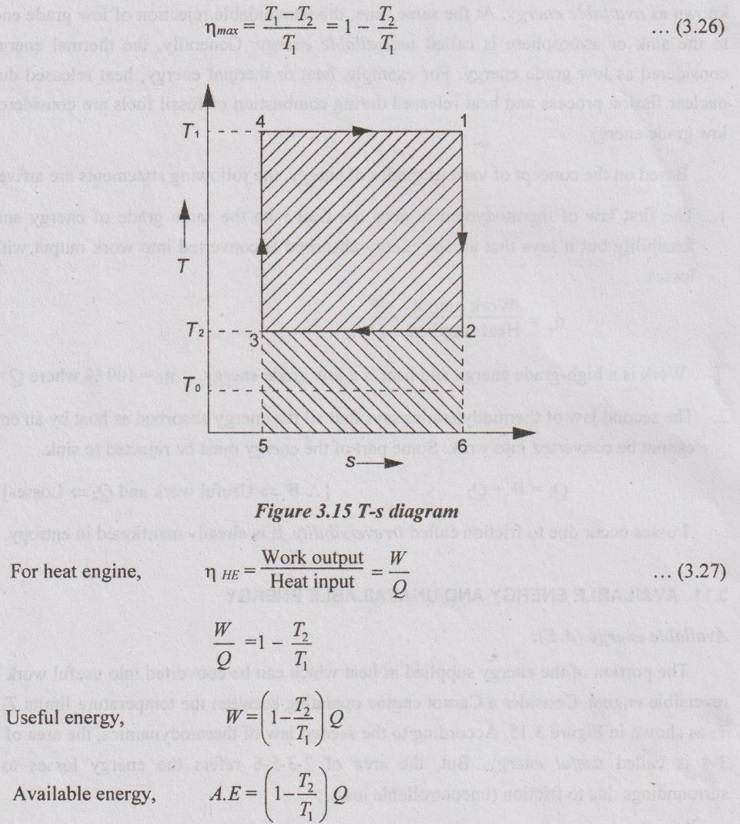 If T2 = To,
If T2 = To,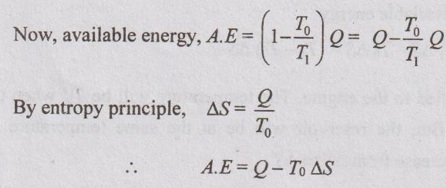
Unavailable energy (U.A.E):

Engineering Thermodynamics: Unit III: Availability and Applications of II Law : Tag: : Thermodynamics - Available Energy and Unavailable Energy
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
