Engineering Thermodynamics: Unit III: Availability and Applications of II Law
Availability
Thermodynamics
A thermodynamic system undergoes a change of state through a reversible process until it comes to equilibrium with the atmosphere.
AVAILABILITY A thermodynamic system undergoes a change of state through a reversible process until it comes to equilibrium with the atmosphere. Then, the work done by the system on the atmosphere is maximum. The maximum work obtained is called availability of system. The system transfers heat to the atmosphere at pressure po and temperature To. Here, the equilibrium will exist between system and atmosphere [po =1.01325 bar and To = 25°C]. It is denoted by ϕ for closed system and ψ (or) B for open system.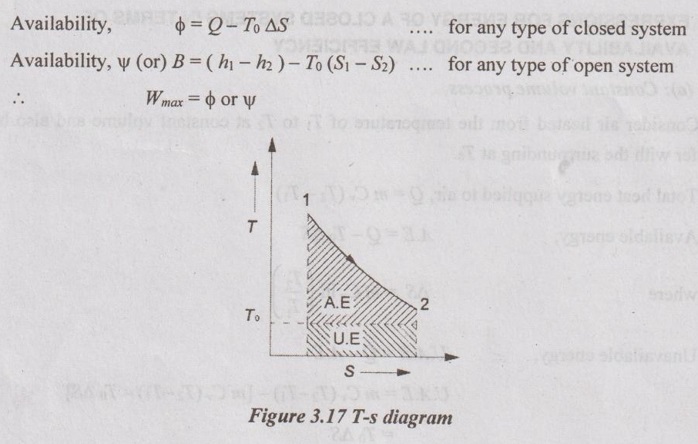
Engineering Thermodynamics: Unit III: Availability and Applications of II Law : Tag: : Thermodynamics - Availability
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
