Engineering Thermodynamics: Unit III: Availability and Applications of II Law
Applications of Change in Entropy for different processes
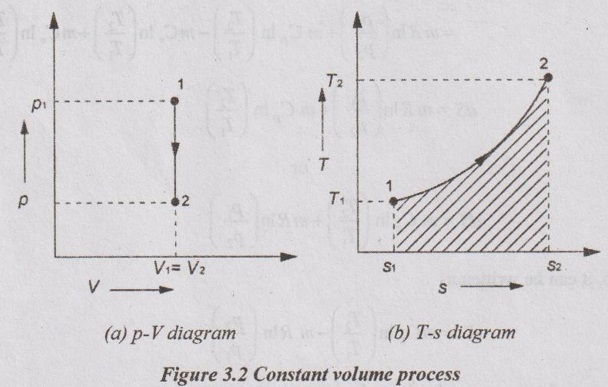
A system undergoing a change of state from 1 to 2 at a constant volume process is shown in p-V and T-s diagram of Figure 3.2.
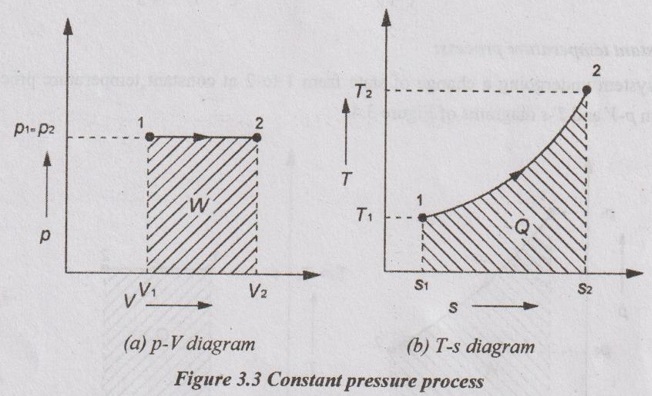
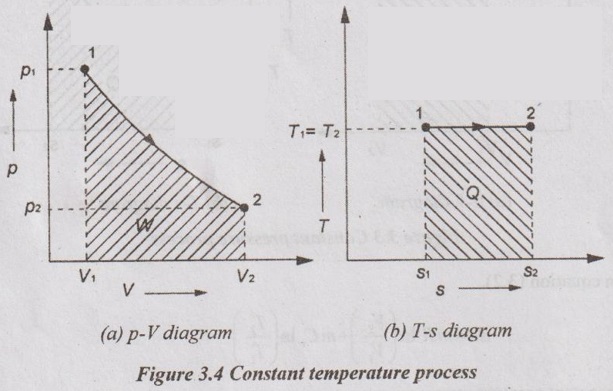
APPLICATIONS OF CHANGE IN ENTROPY FOR DIFFERENT PROCESSES A system undergoing a change of state from 1 to 2 at a constant volume process is shown in p-V and T-s diagram of Figure 3.2. From equation (3.2), the change in entropy is given by But in constant volume process, For constant volume process, A system undergoing a change of state from 1 to 2 at a constant pressure process is shown in p-V and T-s diagrams of Figure 3.3. From equation (3.2), For constant pressure process, A system undergoing a change of state from 1 to 2 at constant temperature process is shown in p-V and T-s diagrams of Figure 3.4. From equation (3.2), Multiplying and dividing by 'T' on right hand side, Adiabatic process is the process in which the heat is neither received nor rejected. So, there is no heat transfer during the process. p-V and T-s diagrams are shown in Figure 3.5. Note: (i) There is no area under T-s diagram because there is no heat transfer. (ii) Since there is no heat transfer during the process, the change in entropy is zero (dS = 0). During polytropic process, there is a heat transfer between a system and its surrounding. There will always be a change in specific entropy. p-V and T-s diagrams for polytropic process are shown in Figure 3.6. We know that from equation (3.2), Change in entropy is calculated by using equation (3.2), (3.8) and (3.7). Another expression may be derived as follows: Heat transfer during polytropic process is given by Dividing by 'T' on both sides, Integrating both sides from initial to final stage,(a) Constant volume process:
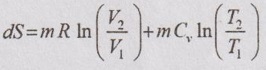
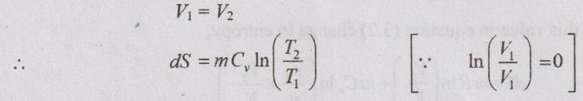
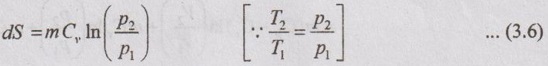
(b) Constant pressure process:
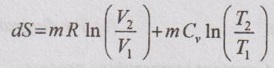
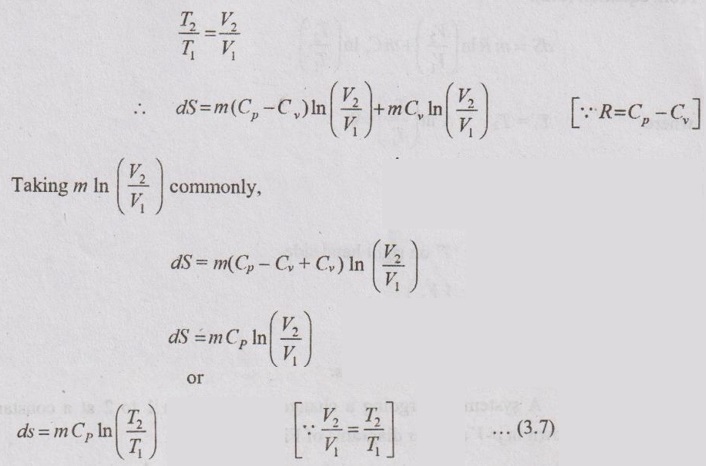
(c) Constant temperature process:
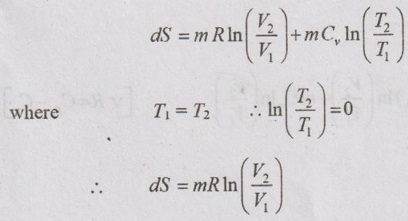
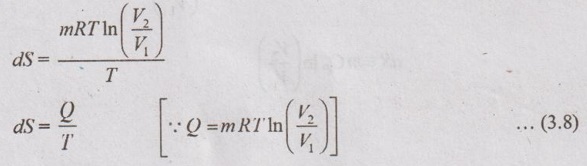
(d) Reversible adiabatic or isentropic process:
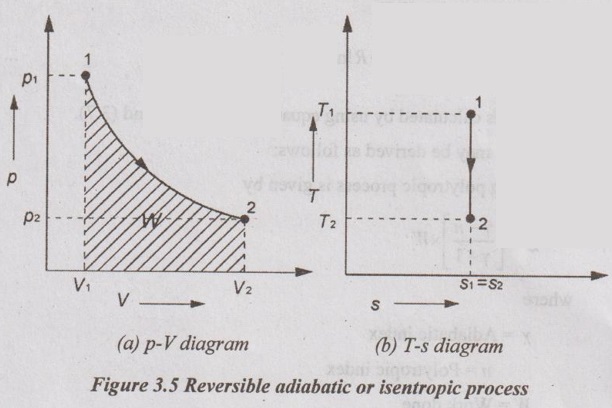
(e) Polytropic process:
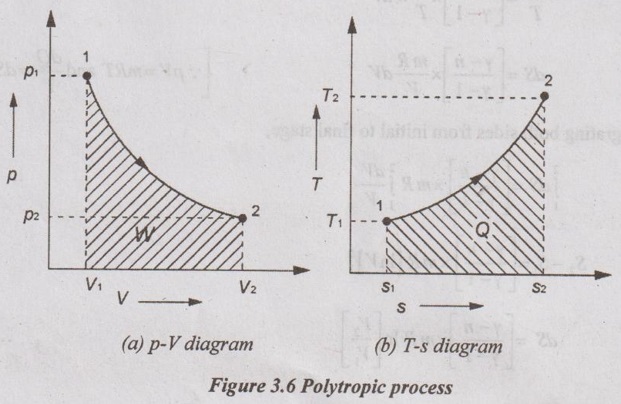


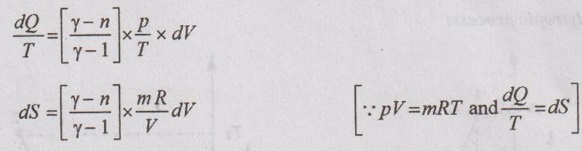
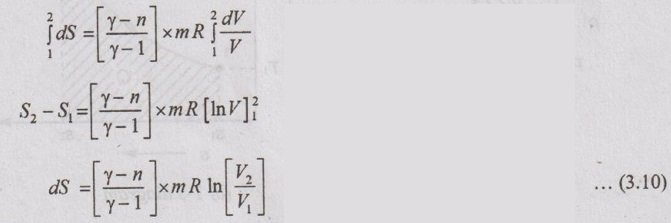 The equation (3.10) is one form of change in entropy. It can be deduced in other way also.
The equation (3.10) is one form of change in entropy. It can be deduced in other way also.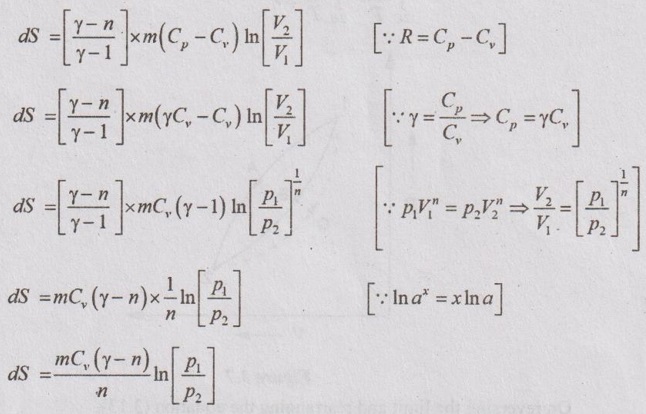
Engineering Thermodynamics: Unit III: Availability and Applications of II Law : Tag: : - Applications of Change in Entropy for different processes
Related Topics
Related Subjects
Engineering Thermodynamics
ME3391 3rd semester Mechanical Dept | 2021 Regulation | 3rd Semester Mechanical Dept 2021 Regulation
